An Improved SHANEL Procedure for Clearing and Staining Brain Tissue from Multiple Species
Abstract
:1. Introduction
2. Results
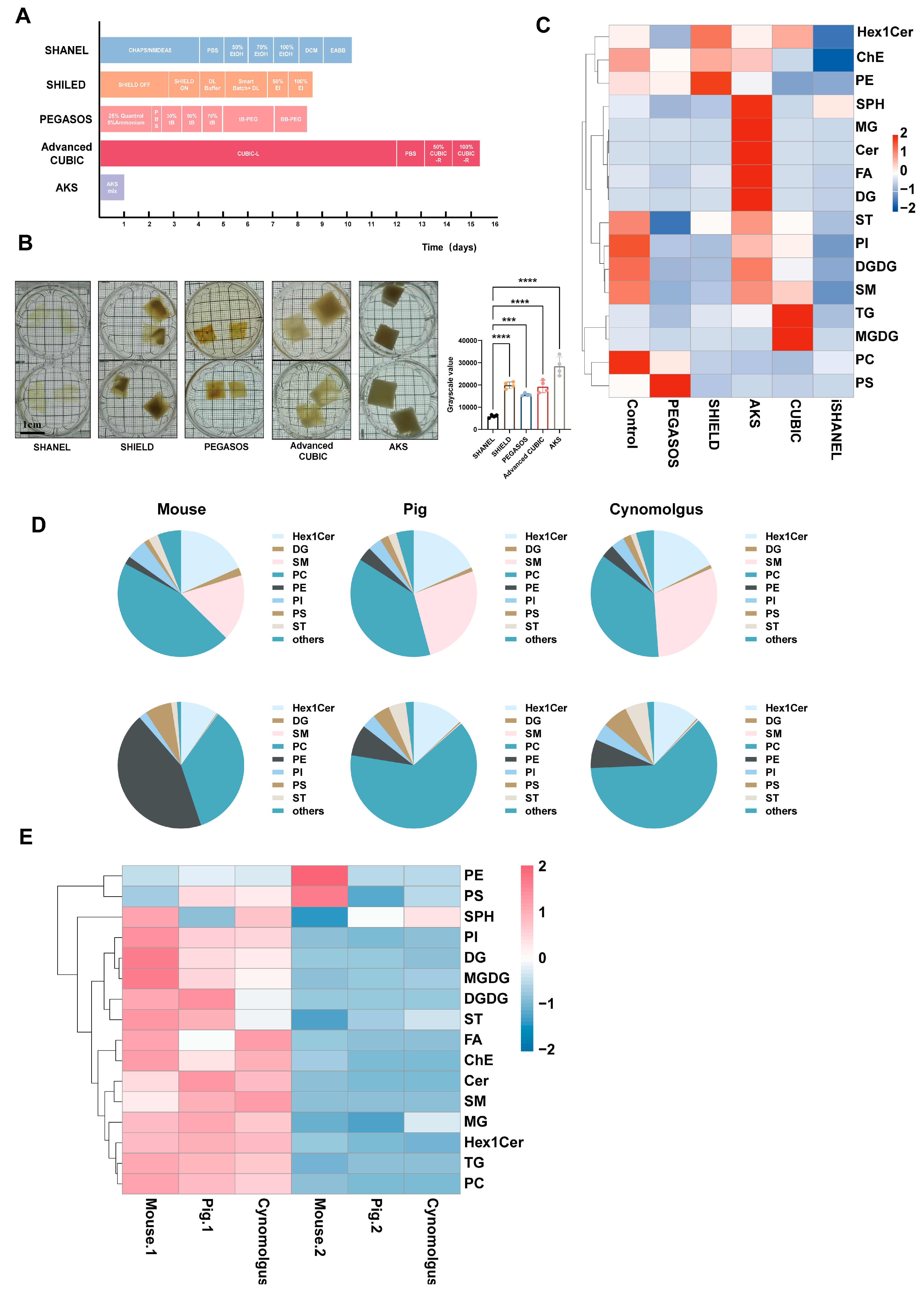
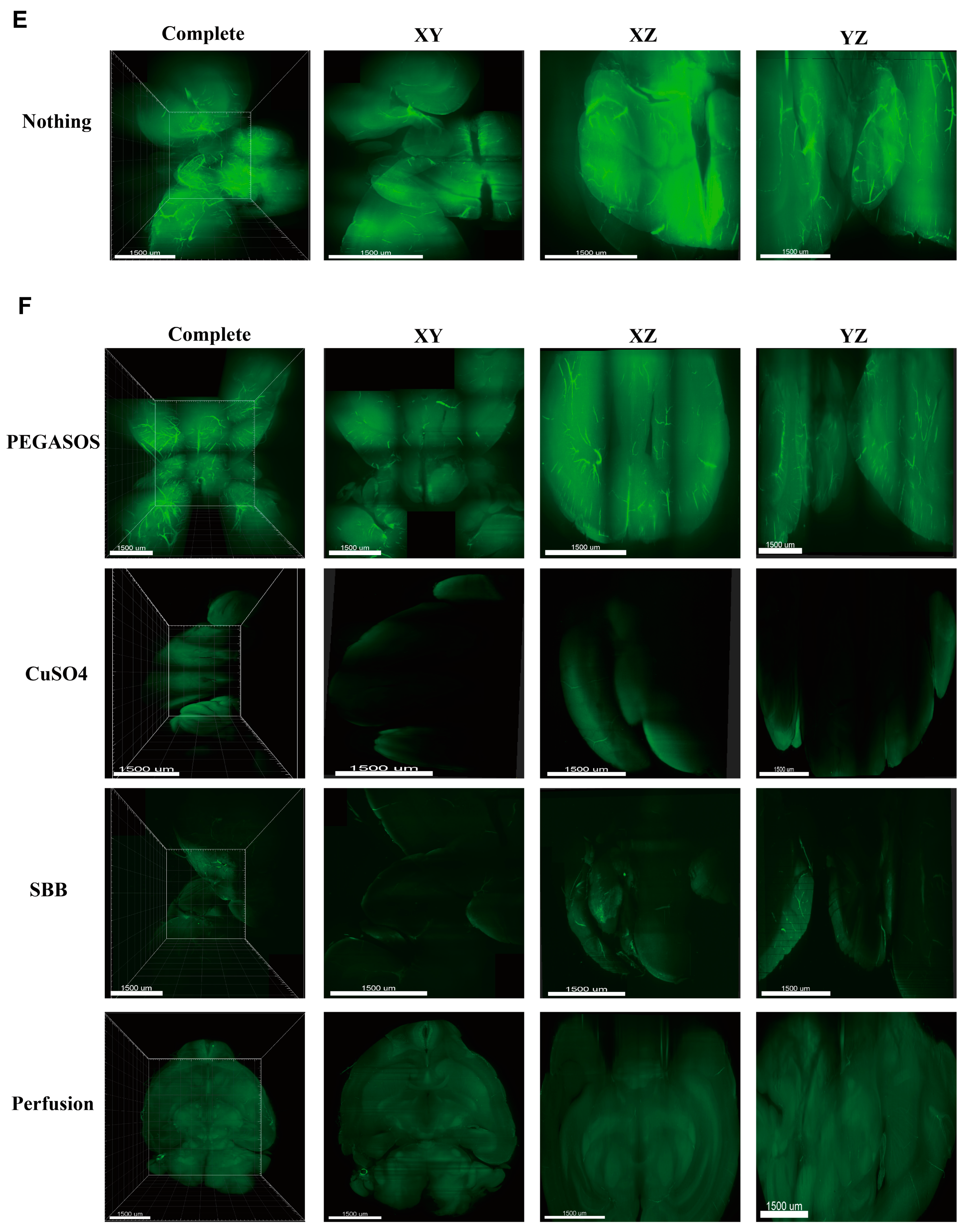
2.1. Comparison of Different Methods for Clearance of Large Brain Tissue
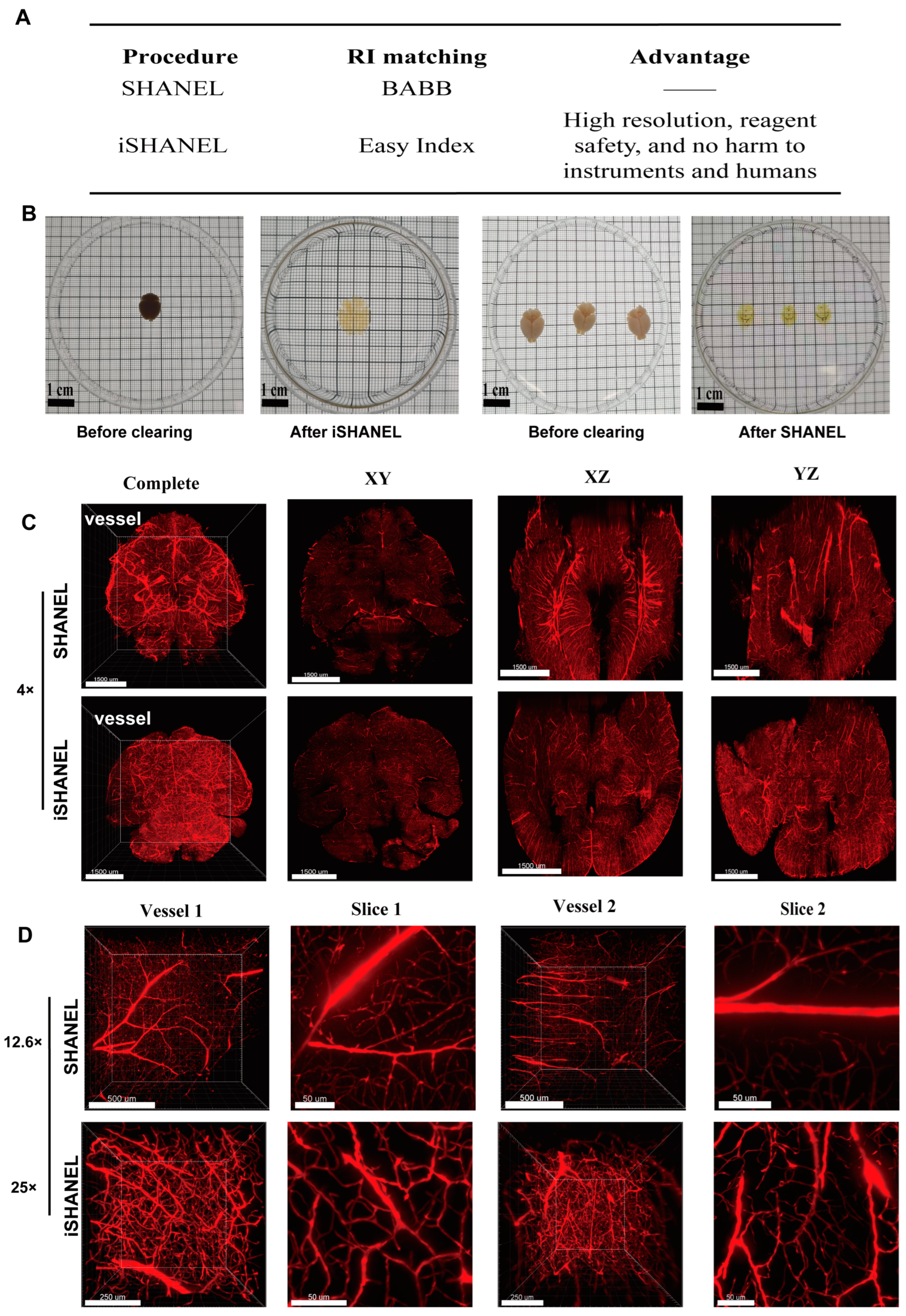
2.2. Clearing Brain Tissue with iSHANEL and 3D Imaging
2.3. Application of iSHANEL Technology to Brain Tissue from Multiple Species
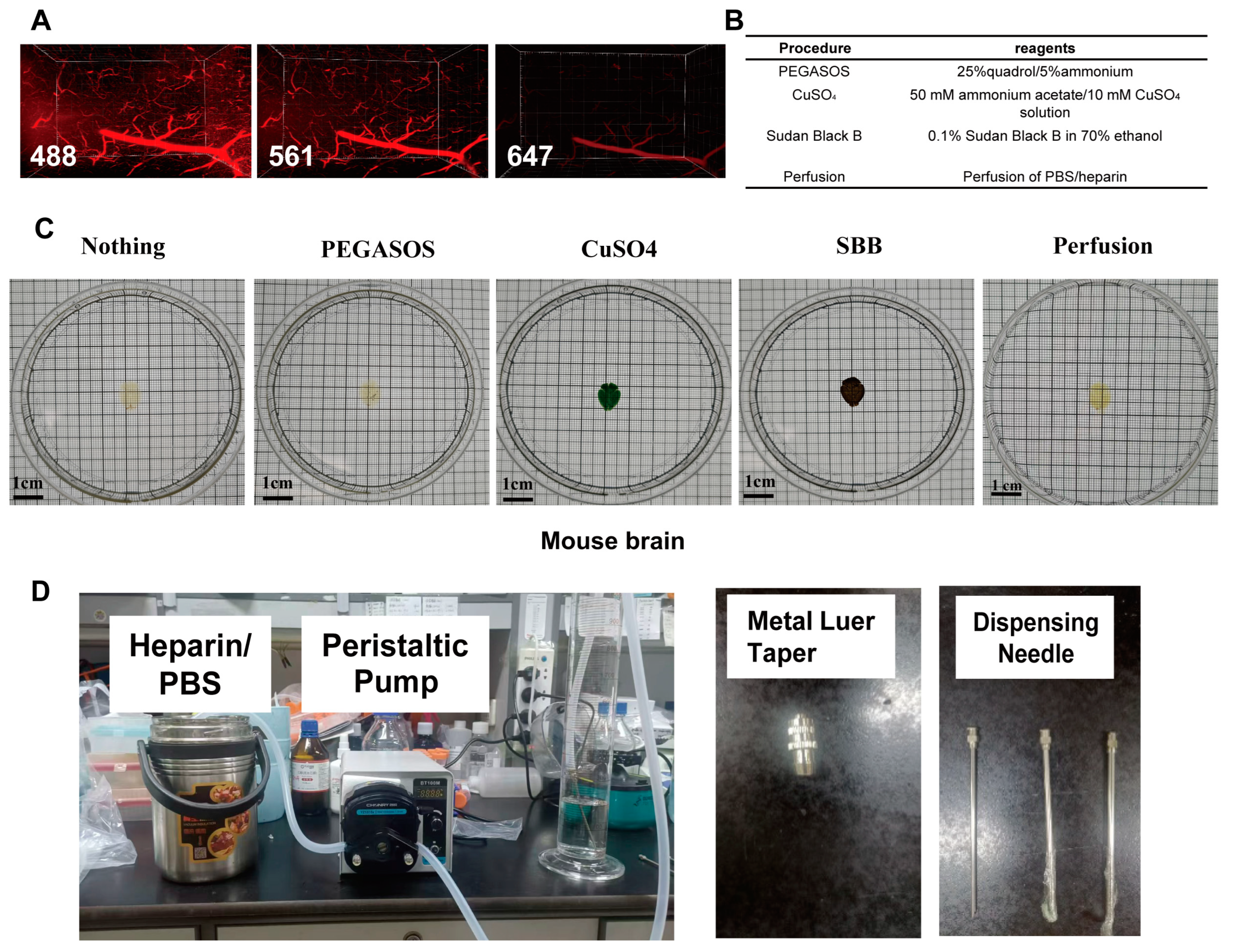
2.4. A New Sample Processing Method to Reduce Blood Vessel Autofluorescence
3. Discussion
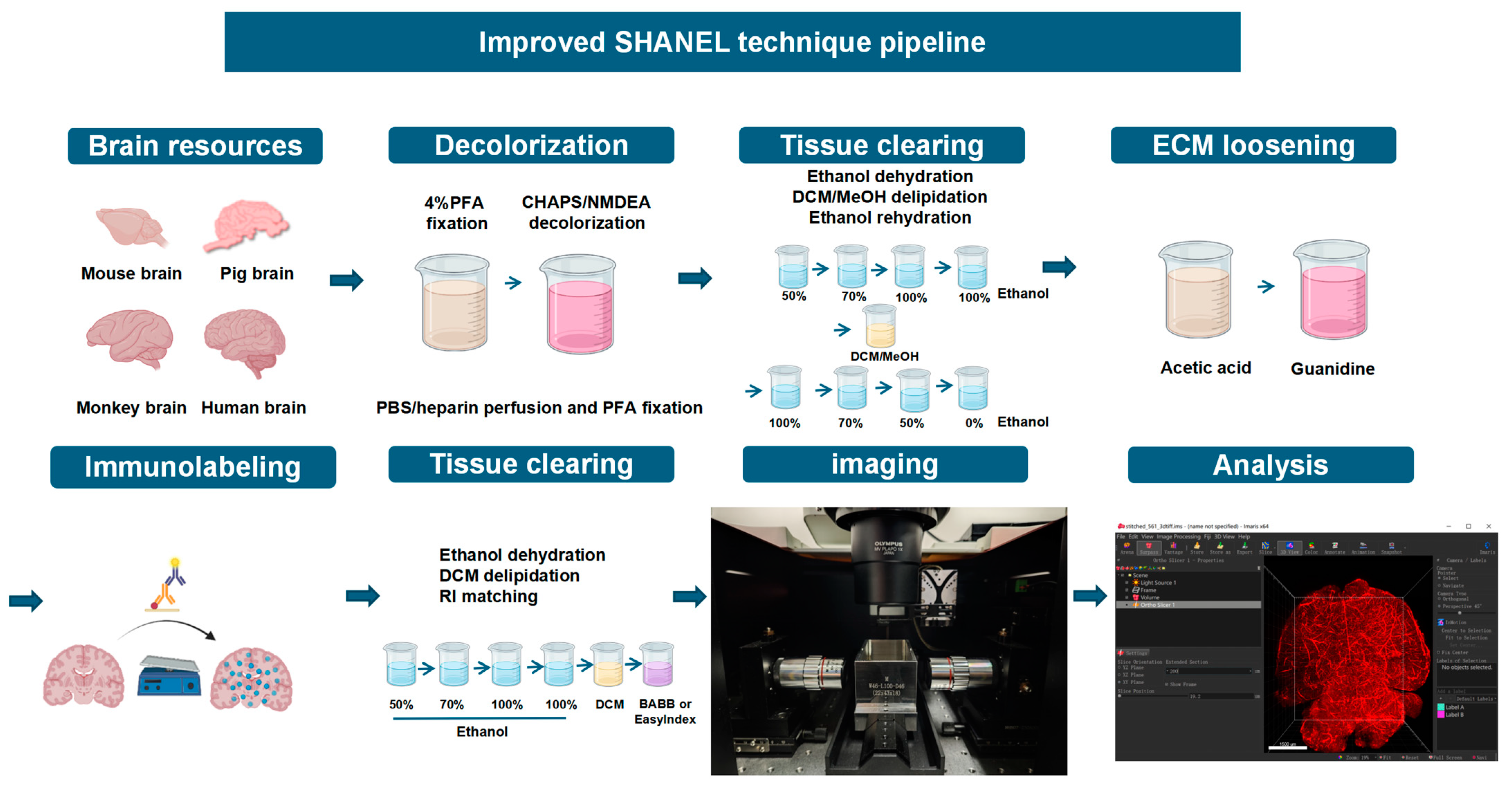
4. Materials and Methods
4.1. Human Subjects and Animals
4.2. Labeling of the Vasculature
4.3. Immunolabeling
4.4. Lipidomics
4.5. Comparison of the Different Tissue Clearing Methods
4.6. Elimination of Tissue Sample Autofluorescence
4.7. Improved Tissue Clearing Method
4.8. Light Sheet Microscopy
4.9. Analysis Methods
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| iSHANEL | Improved SHANEL |
| RI | Refractive index |
| IBA-1 | Ionized Calcium-Binding Adapter Molecule 1 |
| CuSO4 | Cupric sulfate |
| SBB | Sudan Black B |
| CHAPS | 3-[(3-cholamidopropyl) dimethylammonio]-1-propanesulfonate |
| NMDEA | N-methyldiethanolamine |
| DCM | Dichloromethane |
| BABB | Benzyl benzoate–benzyl alcohol (2:1) |
References
- Weiss, K.R.; Voigt, F.F.; Shepherd, D.P.; Huisken, J. Tutorial: Practical Considerations for Tissue Clearing and Imaging. Nat. Protoc. 2021, 16, 2732–2748. [Google Scholar] [CrossRef] [PubMed]
- Ueda, H.R.; Ertürk, A.; Chung, K.; Gradinaru, V.; Chédotal, A.; Tomancak, P.; Keller, P.J. Tissue Clearing and Its Applications in Neuroscience. Nat. Rev. Neurosci. 2020, 21, 61–79. [Google Scholar] [CrossRef] [PubMed]
- Ueda, H.R.; Dodt, H.-U.; Osten, P.; Economo, M.N.; Chandrashekar, J.; Keller, P.J. Whole-Brain Profiling of Cells and Circuits in Mammals by Tissue Clearing and Light-Sheet Microscopy. Neuron 2020, 106, 369–387. [Google Scholar] [CrossRef]
- Tainaka, K.; Murakami, T.C.; Susaki, E.A.; Shimizu, C.; Saito, R.; Takahashi, K.; Hayashi-Takagi, A.; Sekiya, H.; Arima, Y.; Nojima, S.; et al. Chemical Landscape for Tissue Clearing Based on Hydrophilic Reagents. Cell Rep. 2018, 24, 2196–2210.e9. [Google Scholar] [CrossRef]
- Chen, F.; Tillberg, P.W.; Boyden, E.S. Expansion microscopy. Science 2015, 347, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Vigouroux, R.J.; Belle, M.; Chédotal, A. Neuroscience in the Third Dimension: Shedding New Light on the Brain with Tissue Clearing. Mol. Brain 2017, 10, 33. [Google Scholar] [CrossRef]
- Susaki, E.A.; Tainaka, K.; Perrin, D.; Yukinaga, H.; Kuno, A.; Ueda, H.R. Advanced CUBIC Protocols for Whole-Brain and Whole-Body Clearing and Imaging. Nat. Protoc. 2015, 10, 1709–1727. [Google Scholar] [CrossRef]
- Cai, R.; Kolabas, Z.I.; Pan, C.; Mai, H.; Zhao, S.; Kaltenecker, D.; Voigt, F.F.; Molbay, M.; Ohn, T.-L.; Vincke, C.; et al. Whole-Mouse Clearing and Imaging at the Cellular Level with vDISCO. Nat. Protoc. 2023, 18, 1197–1242. [Google Scholar] [CrossRef]
- Cai, R.; Pan, C.; Ghasemigharagoz, A.; Todorov, M.I.; Förstera, B.; Zhao, S.; Bhatia, H.S.; Parra-Damas, A.; Mrowka, L.; Theodorou, D.; et al. Panoptic Imaging of Transparent Mice Reveals Whole-Body Neuronal Projections and Skull-Meninges Connections. Nat. Neurosci. 2019, 22, 317–327. [Google Scholar] [CrossRef]
- Lai, H.M.; Ng, W.-L.; Gentleman, S.M.; Wu, W. Chemical Probes for Visualizing Intact Animal and Human Brain Tissue. Cell Chem. Biol. 2017, 24, 659–672. [Google Scholar] [CrossRef]
- Lai, H.M.; Liu, A.K.L.; Ng, H.H.M.; Goldfinger, M.H.; Chau, T.W.; DeFelice, J.; Tilley, B.S.; Wong, W.M.; Wu, W.; Gentleman, S.M. Next Generation Histology Methods for Three-Dimensional Imaging of Fresh and Archival Human Brain Tissues. Nat. Commun. 2018, 9, 1066. [Google Scholar] [CrossRef] [PubMed]
- Costantini, I.; Baria, E.; Sorelli, M.; Matuschke, F.; Giardini, F.; Menzel, M.; Mazzamuto, G.; Silvestri, L.; Cicchi, R.; Amunts, K.; et al. Autofluorescence Enhancement for Label-Free Imaging of Myelinated Fibers in Mammalian Brains. Sci. Rep. 2021, 11, 8038. [Google Scholar] [CrossRef] [PubMed]
- Terman, A.; Brunk, U.T. Lipofuscin: Mechanisms of Formation and Increase with Age. APMIS 1998, 106, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Werner, M.; Chott, A.; Fabiano, A.; Battifora, H. Effect of Formalin Tissue Fixation and Processing on Immunohistochemistry. Am. J. Surg. Pathol. 2000, 24, 1016–1019. [Google Scholar] [CrossRef]
- Tainaka, K.; Kuno, A.; Kubota, S.I.; Murakami, T.; Ueda, H.R. Chemical Principles in Tissue Clearing and Staining Protocols for Whole-Body Cell Profiling. Annu. Rev. Cell Dev. Biol. 2016, 32, 713–741. [Google Scholar] [CrossRef]
- Susaki, E.A.; Ueda, H.R. Whole-Body and Whole-Organ Clearing and Imaging Techniques with Single-Cell Resolution: Toward Organism-Level Systems Biology in Mammals. Cell Chem. Biol. 2016, 23, 137–157. [Google Scholar] [CrossRef]
- Bozek, K.; Wei, Y.; Yan, Z.; Liu, X.; Xiong, J.; Sugimoto, M.; Tomita, M.; Pääbo, S.; Sherwood, C.C.; Hof, P.R.; et al. Organization and Evolution of Brain Lipidome Revealed by Large-Scale Analysis of Human, Chimpanzee, Macaque, and Mouse Tissues. Neuron 2015, 85, 695–702. [Google Scholar] [CrossRef]
- Zhan, Y.; Wu, H.; Liu, L.; Lin, J.; Zhang, S. Organic Solvent-Based Tissue Clearing Techniques and Their Applications. J. Biophotonics 2021, 14, e202000413. [Google Scholar] [CrossRef]
- Tainaka, K.; Kubota, S.I.; Suyama, T.Q.; Susaki, E.A.; Perrin, D.; Ukai-Tadenuma, M.; Ukai, H.; Ueda, H.R. Whole-body imaging with single-cell resolution by tissue decolorization. Cell 2014, 159, 911–924. [Google Scholar] [CrossRef]
- Stadelmann, C.; Timmler, S.; Barrantes-Freer, A.; Simons, M. Myelin in the Central Nervous System: Structure, Function, and Pathology. Physiol. Rev. 2019, 99, 1381–1431. [Google Scholar] [CrossRef]
- Peters, A. The Effects of Normal Aging on Myelin and Nerve Fibers: A Review. J. Neurocytol. 2002, 31, 581–593. [Google Scholar] [CrossRef] [PubMed]
- Olsen, A.S.B.; Færgeman, N.J. Sphingolipids: Membrane Microdomains in Brain Development, Function and Neurological Diseases. Open Biol. 2017, 7, 170069. [Google Scholar] [CrossRef]
- Susaki, E.A.; Tainaka, K.; Perrin, D.; Kishino, F.; Tawara, T.; Watanabe, T.M.; Yokoyama, C.; Onoe, H.; Eguchi, M.; Yamaguchi, S.; et al. Whole-Brain Imaging with Single-Cell Resolution Using Chemical Cocktails and Computational Analysis. Cell 2014, 157, 726–739. [Google Scholar] [CrossRef] [PubMed]
- Sjöstedt, E.; Zhong, W.; Fagerberg, L.; Karlsson, M.; Mitsios, N.; Adori, C.; Oksvold, P.; Edfors, F.; Limiszewska, A.; Hikmet, F.; et al. An Atlas of the Protein-Coding Genes in the Human, Pig, and Mouse Brain. Science 2020, 367, eaay5947. [Google Scholar] [CrossRef]
- Zhao, S.; Todorov, M.I.; Cai, R.; Maskari, R.A.; Steinke, H.; Kemter, E.; Mai, H.; Rong, Z.; Warmer, M.; Stanic, K.; et al. Cellular and Molecular Probing of Intact Human Organs. Cell 2020, 180, 796–812.e19. [Google Scholar] [CrossRef]
- Kim, S.-Y.; Cho, J.H.; Murray, E.; Bakh, N.; Choi, H.; Ohn, K.; Ruelas, L.; Hubbert, A.; McCue, M.; Vassallo, S.L.; et al. Stochastic Electrotransport Selectively Enhances the Transport of Highly Electromobile Molecules. Proc. Natl. Acad. Sci. USA 2015, 112, E6274–E6283. [Google Scholar] [CrossRef] [PubMed]
- Na, M.; Kim, K.; Oh, K.; Choi, H.J.; Ha, C.; Chang, S. Sodium Cholate-Based Active Delipidation for Rapid and Efficient Clearing and Immunostaining of Deep Biological Samples. Small Methods 2021, 6, 2100943. [Google Scholar] [CrossRef]
- Glaser, A.K.; Bishop, K.W.; Barner, L.A.; Susaki, E.A.; Kubota, S.I.; Gao, G.; Serafin, R.B.; Balaram, P.; Turschak, E.; Nicovich, P.R.; et al. A Hybrid Open-Top Light-Sheet Microscope for Versatile Multi-Scale Imaging of Cleared Tissues. Nat. Methods 2022, 19, 613–619. [Google Scholar] [CrossRef]
- Vladimirov, N.; Voigt, F.F.; Naert, T.; Araujo, G.R.; Cai, R.; Reuss, A.M.; Zhao, S.; Schmid, P.; Hildebrand, S.; Schaettin, M.; et al. Benchtop mesoSPIM: A next-Generation Open-Source Light-Sheet Microscope for Cleared Samples. Nat. Commun. 2024, 15, 2679. [Google Scholar] [CrossRef]
- Deng, Y.; Zhu, J.; Liu, X.; Dai, J.; Yu, T.; Zhu, D. A Robust Vessel-Labeling Pipeline with High Tissue Clearing Compatibility for 3D Mapping of Vascular Networks. iScience 2024, 27, 109730. [Google Scholar] [CrossRef]
- Barchuk, M.; Dutour, A.; Ancel, P.; Svilar, L.; Miksztowicz, V.; Lopez, G.; Rubio, M.; Schreier, L.; Nogueira, J.P.; Valéro, R.; et al. Untargeted Lipidomics Reveals a Specific Enrichment in Plasmalogens in Epicardial Adipose Tissue and a Specific Signature in Coronary Artery Disease. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 986–1000. [Google Scholar] [CrossRef] [PubMed]
- Matyash, V.; Liebisch, G.; Kurzchalia, T.V.; Shevchenko, A.; Schwudke, D. Lipid Extraction by Methyl-Tert-Butyl Ether for High-Throughput Lipidomics. J. Lipid Res. 2008, 49, 1137–1146. [Google Scholar] [CrossRef] [PubMed]
- Giera, M. (Ed.) Clinical Metabolomics: Methods and Protocols; Methods in Molecular Biology; Springer: New York, NY, USA, 2018; Volume 1730, ISBN 978-1-4939-7591-4. [Google Scholar]
- Mai, H.; Rong, Z.; Zhao, S.; Cai, R.; Steinke, H.; Bechmann, I.; Ertürk, A. Scalable Tissue Labeling and Clearing of Intact Human Organs. Nat. Protoc. 2022, 17, 2188–2215. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.-G.; Sohn, C.H.; Chen, R.; McCue, M.; Yun, D.H.; Drummond, G.T.; Ku, T.; Evans, N.B.; Oak, H.C.; Trieu, W.; et al. Protection of Tissue Physicochemical Properties Using Polyfunctional Crosslinkers. Nat. Biotechnol. 2018, 37, 73–83. [Google Scholar] [CrossRef]
- Jing, D.; Zhang, S.; Luo, W.; Gao, X.; Men, Y.; Ma, C.; Liu, X.; Yi, Y.; Bugde, A.; Zhou, B.O.; et al. Author Correction: Tissue Clearing of Both Hard and Soft Tissue Organs with the PEGASOS Method. Cell Res. 2019, 29, 506. [Google Scholar] [CrossRef]
- Shan, Q.-H.; Qin, X.-Y.; Zhou, N.; Huang, C.; Wang, Y.; Chen, P.; Zhou, J.-N. A Method for Ultrafast Tissue Clearing That Preserves Fluorescence for Multimodal and Longitudinal Brain Imaging. BMC Biol. 2022, 20, 77. [Google Scholar] [CrossRef]
- Oliveira, V.C.; Carrara, R.C.V.; Simoes, D.L.C.; Saggioro, F.P.; Carlotti, C.G.; Covas, D.T.; Neder, L. Sudan Black B Treatment Reduces Autofluorescence and Improves Resolution of in Situ Hybridization Specific Fluorescent Signals of Brain Sections. Histol. Histopathol. 2010, 25, 1017–1024. [Google Scholar] [CrossRef]
- Kaltenecker, D.; Al-Maskari, R.; Negwer, M.; Hoeher, L.; Kofler, F.; Zhao, S.; Todorov, M.; Rong, Z.; Paetzold, J.C.; Wiestler, B.; et al. Virtual Reality-Empowered Deep-Learning Analysis of Brain Cells. Nat. Methods 2024, 21, 1306–1315. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, F.; Huang, L.; Han, D.; Liu, R.; Zhang, K.; Su, Y.; Su, T.; Lin, Y.; Xiu, J. An Improved SHANEL Procedure for Clearing and Staining Brain Tissue from Multiple Species. Int. J. Mol. Sci. 2025, 26, 3569. https://doi.org/10.3390/ijms26083569
Zeng F, Huang L, Han D, Liu R, Zhang K, Su Y, Su T, Lin Y, Xiu J. An Improved SHANEL Procedure for Clearing and Staining Brain Tissue from Multiple Species. International Journal of Molecular Sciences. 2025; 26(8):3569. https://doi.org/10.3390/ijms26083569
Chicago/Turabian StyleZeng, Fu, Lian Huang, Ding Han, Renjie Liu, Kongjia Zhang, Yuwen Su, Tong Su, Yarong Lin, and Jianbo Xiu. 2025. "An Improved SHANEL Procedure for Clearing and Staining Brain Tissue from Multiple Species" International Journal of Molecular Sciences 26, no. 8: 3569. https://doi.org/10.3390/ijms26083569
APA StyleZeng, F., Huang, L., Han, D., Liu, R., Zhang, K., Su, Y., Su, T., Lin, Y., & Xiu, J. (2025). An Improved SHANEL Procedure for Clearing and Staining Brain Tissue from Multiple Species. International Journal of Molecular Sciences, 26(8), 3569. https://doi.org/10.3390/ijms26083569





