Transcriptomic Profile of Human Osteoblast-like Cells Grown on Trabecular Titanium
Abstract
:1. Introduction
2. Results
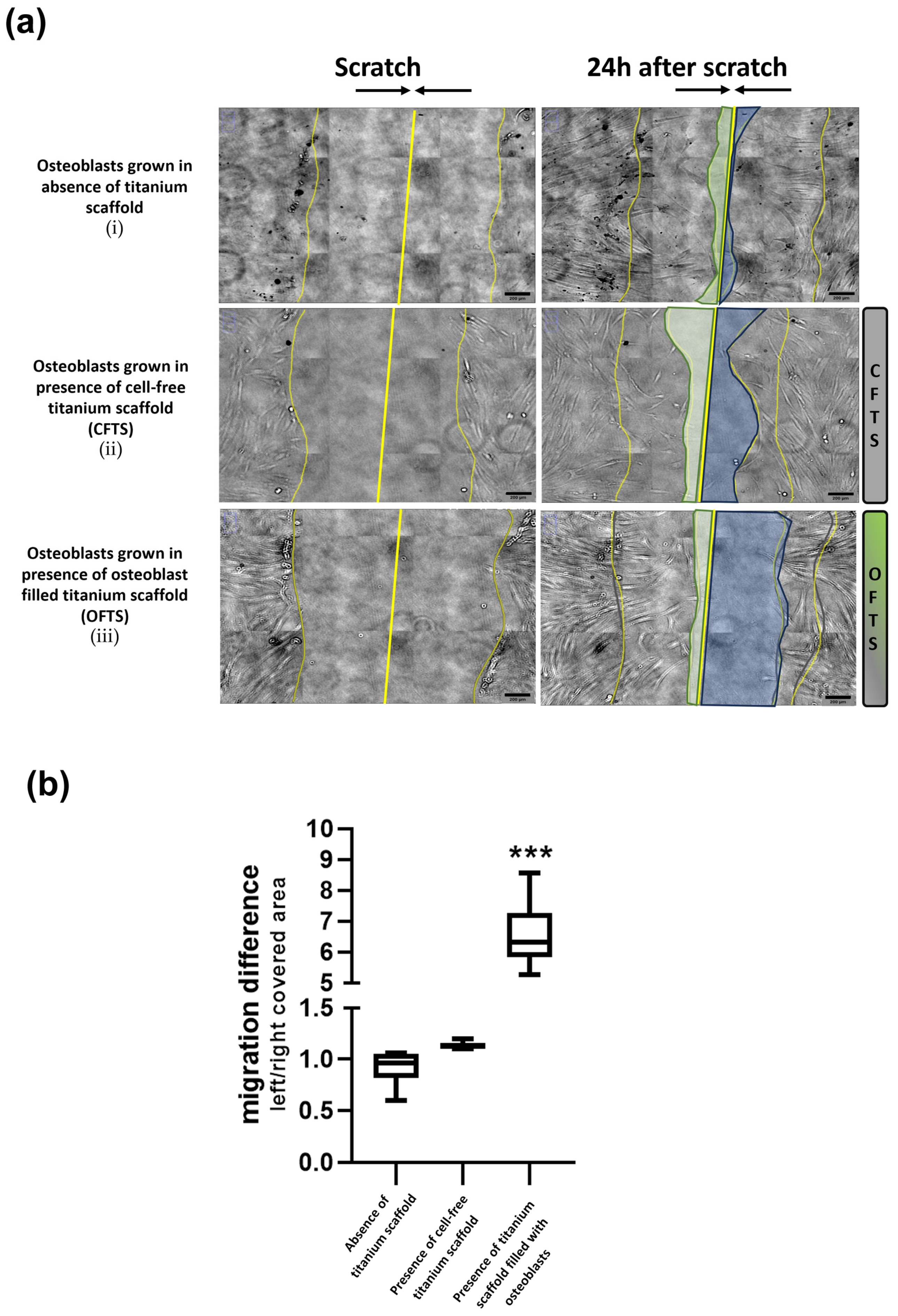
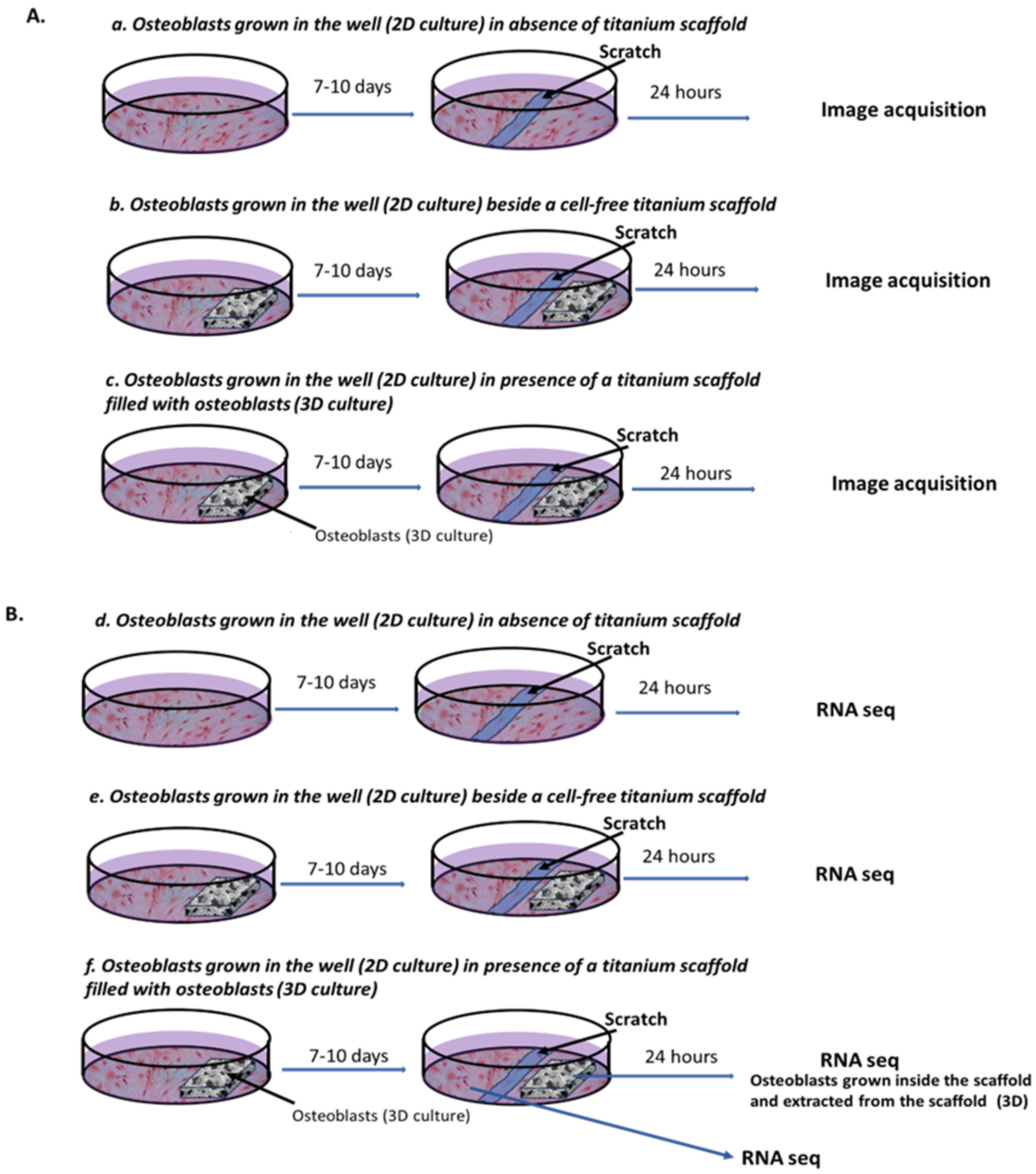
2.1. Effect on Cell Migration of Osteoblasts-Filled Titanium Scaffold
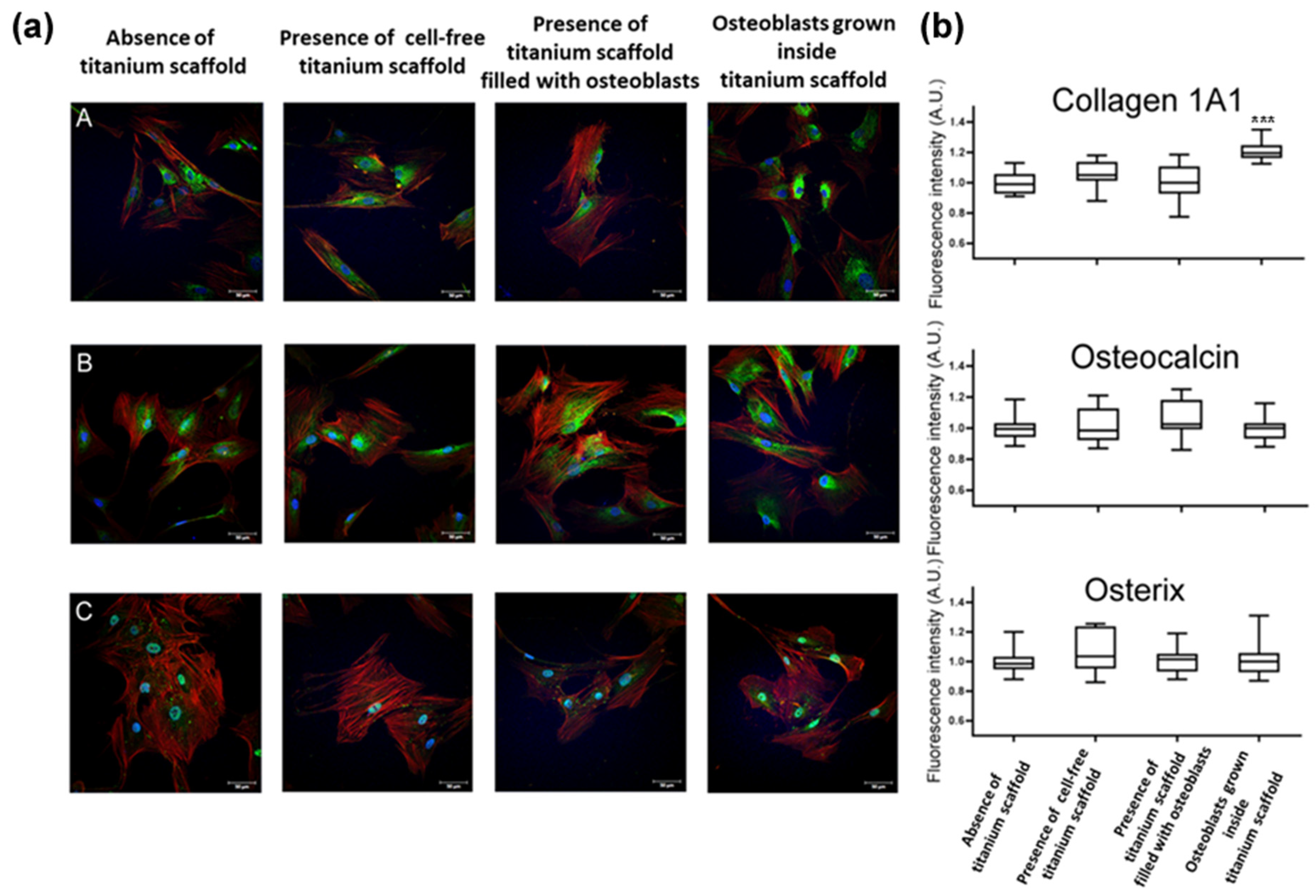
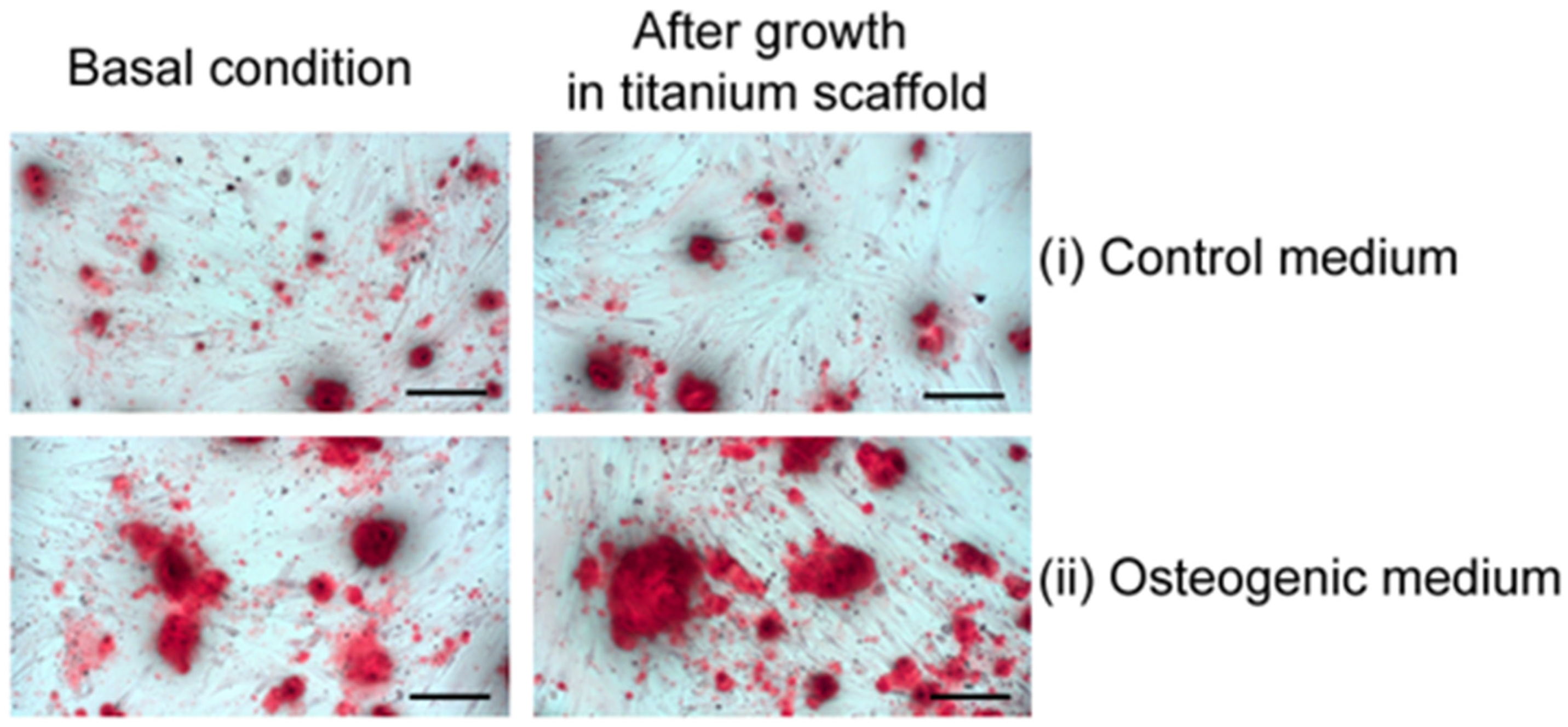
2.2. Primary Osteoblasts Grown Inside Titanium Scaffold Maintain Osteoblast Phenotype, Mineralization Capacity, and Increase Collagen-1A1 Expression
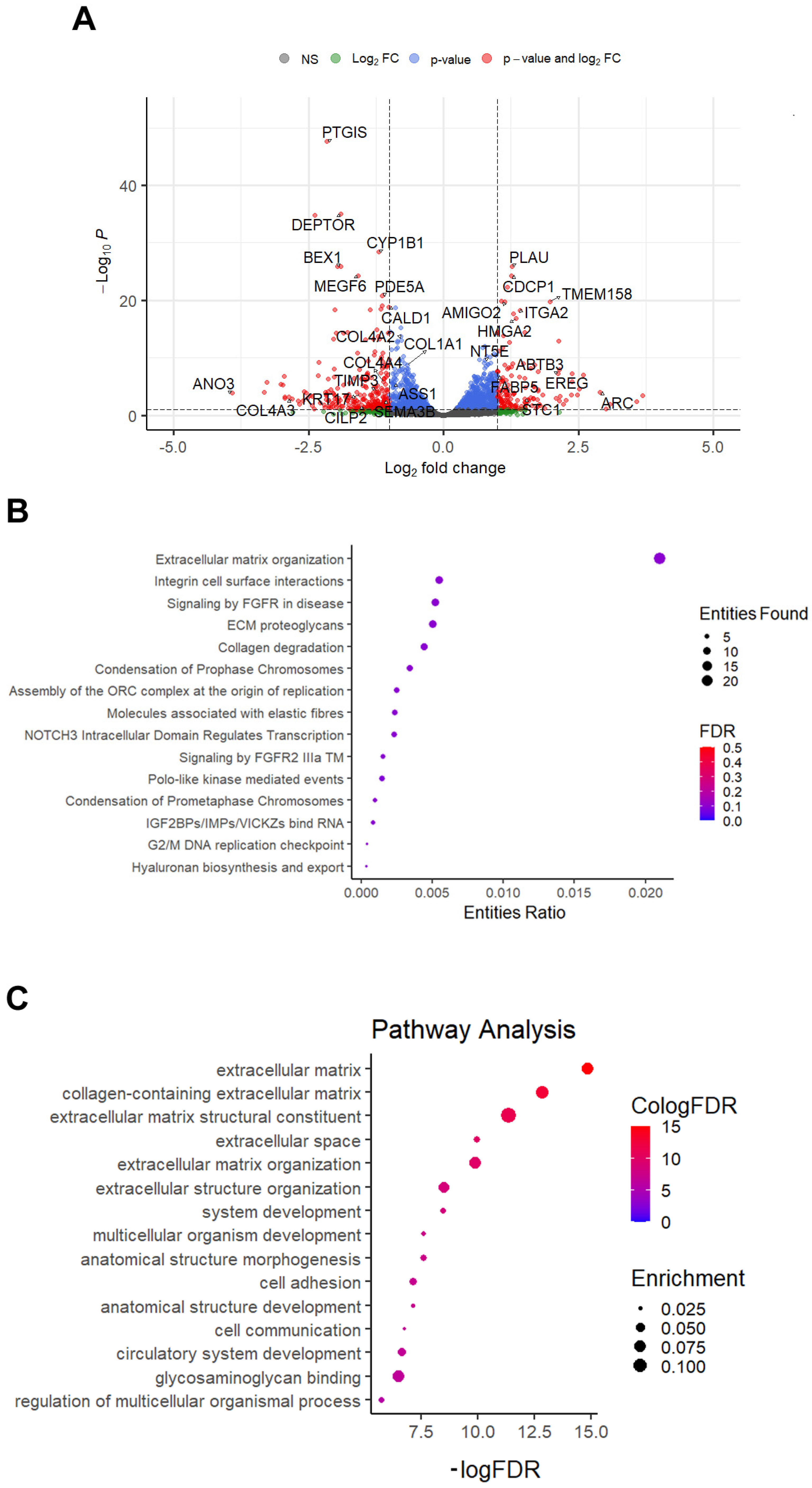
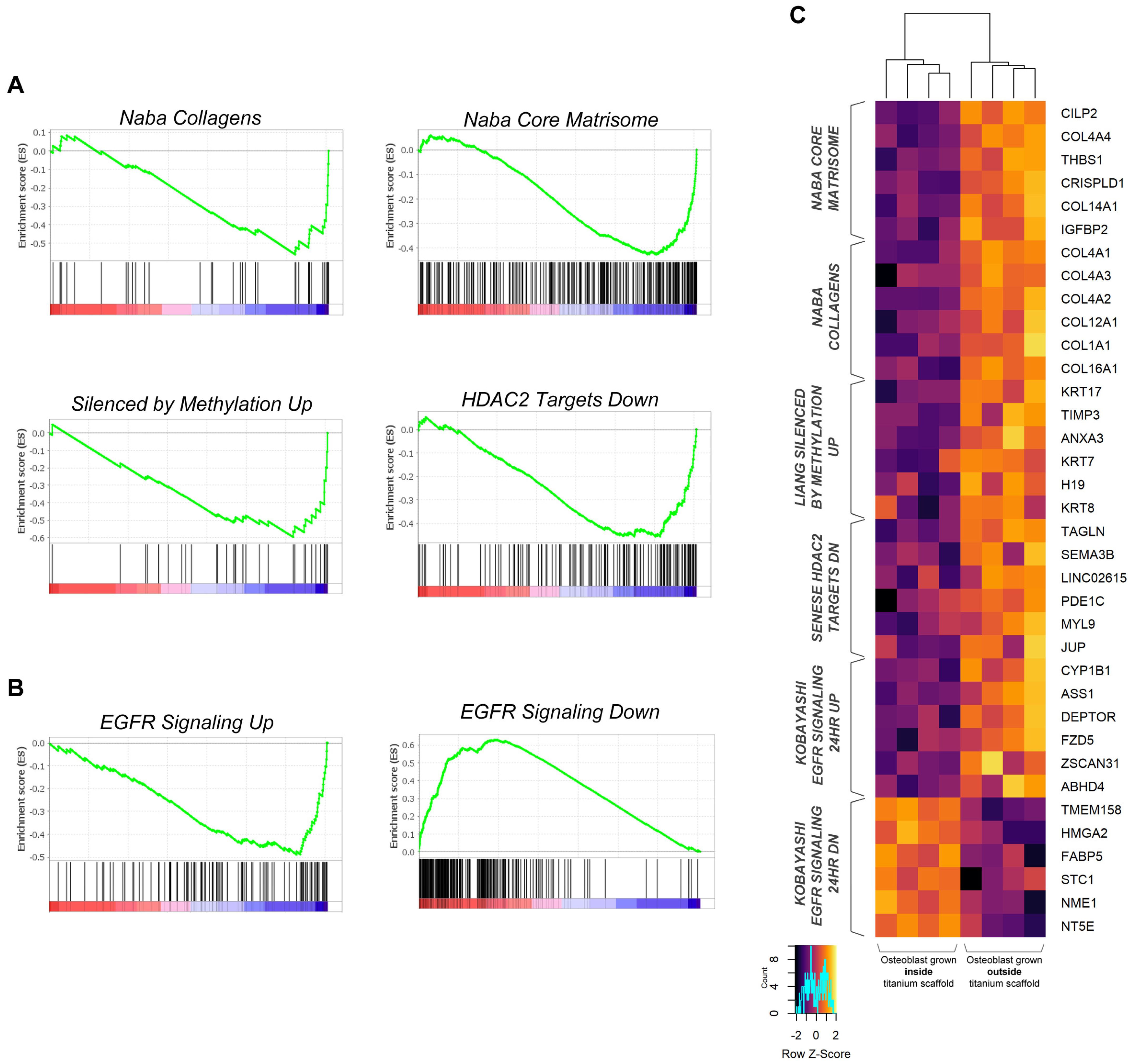
2.3. Primary Osteoblasts Grown Inside Titanium Scaffold Modify Their Transcriptomic Profile
3. Discussion
4. Materials and Methods
4.1. Titanium Scaffolds
4.2. Primary Human Osteoblast Cultures
4.3. Experimental Design
4.4. Immunofluorescence Analysis and Alizarin Stain
4.5. RNA Extraction, Sequencing, and Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Ti | titanium |
| ECM | extracellular matrix |
| DEG | differentially expressed genes |
| GSEA | gene set enrichment analysis |
| CPDB | ConsensusPathDB-human |
| HDAC2 | histone deacetylase 2 |
| EGFR | epidermal growth factor receptor |
| EGF | epidermal growth factor |
| EREG | epiregulin |
| BMP | bone morphogenetic protein |
| RUNX2 | runt-related transcription factor 2 |
| OSX | osterix |
| WNT | wingless-related integration site family |
| FGF | fibroblast growth factor |
| FGFR | fibroblast growth factor receptor |
References
- Huang, C.-H.; Huang, K.-T.; Lee, T.-M. The biological responses of osteoblasts on titanium: Effect of oxygen level and surface roughness. J. Formos. Med. Assoc. 2023, 122, 584–592. [Google Scholar] [CrossRef] [PubMed]
- Kurosaka, M.; Kataoka, Y.; Miyazaki, T. In vitro characterization of primary osteoblasts on titanium surfaces processed with wire-type electric discharge machining. Dent. Mater. J. 2022, 41, 655–659. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhou, P.; Liu, S.; Attarilar, S.; Ma, R.L.; Zhong, Y.; Wang, L. Multi-Scale Surface Treatments of Titanium Implants for Rapid Osseointegration: A Review. Nanomaterials 2020, 10, 1244. [Google Scholar] [CrossRef]
- Komatsu, K.; Matsuura, T.; Suzumura, T.; Ogawa, T. Genome-wide transcriptional responses of osteoblasts to different titanium surface topographies. Mater. Today Bio 2023, 23, 100852. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Singh, K. Review on titanium and titanium based alloys as biomaterials for orthopaedic applications. Mater. Sci. Eng. C 2019, 102, 844–862. [Google Scholar] [CrossRef]
- Svehla, M.; Morberg, P.; Zicat, B.; Bruce, W.; Sonnabend, D.; Walsh, W.R. Morphometric and mechanical evaluation of titanium implant integration: Comparison of five surface structures. J. Biomed. Mater. Res. 2000, 51, 15–22. [Google Scholar] [CrossRef]
- Oliver-Urrutia, C.; Kashimbetova, A.; Slámečka, K.; Casas-Luna, M.; Matula, J.; Sumbalova Koledova, Z.; Kaiser, J.; Čelko, L.; Montufar, E.B. Porous titanium/hydroxyapatite interpenetrating phase composites with optimal mechanical and biological properties for personalized bone repair. Biomater. Adv. 2025, 166, 214079. [Google Scholar] [CrossRef]
- Lu, X.; Wu, Z.; Xu, K.; Wang, X.; Wang, S.; Qiu, H.; Li, X.; Chen, J. Multifunctional Coatings of Titanium Implants Toward Promoting Osseointegration and Preventing Infection: Recent Developments. Front. Bioeng. Biotechnol. 2021, 9, 783816. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, C.; Xie, Q.; Xia, L.; Liu, L.; Bao, W.; Lin, H.; Xiong, X.; Zhang, H.; Zheng, Z.; et al. Recent advances in layer-by-layer assembly scaffolds for co-delivery of bioactive molecules for bone regeneration: An updated review. J. Transl. Med. 2024, 22, 1001. [Google Scholar] [CrossRef]
- Feroz, S.; Cathro, P.; Ivanovski, S.; Muhammad, N. Biomimetic bone grafts and substitutes: A review of recent advancements and applications. Biomed. Eng. Adv. 2023, 6, 100107. [Google Scholar] [CrossRef]
- di Gioia, C.R.; van de Greef, W.M.; Sperti, G.; Castoldi, G.; Todaro, N.; Ierardi, C.; Pieruzzi, F.; Stella, A. Angiotensin II increases calponin expression in cultured rat vascular smooth muscle cells. Biochem. Biophys. Res. Commun. 2000, 279, 965–969. [Google Scholar] [CrossRef]
- Castoldi, G.; di Gioia, C.; Giollo, F.; Carletti, R.; Bombardi, C.; Antoniotti, M.; Roma, F.; Zerbini, G.; Stella, A. Different regulation of miR-29a-3p in glomeruli and tubules in an experimental model of angiotensin II-dependent hypertension: Potential role in renal fibrosis. Clin. Exp. Pharmacol. Physiol. 2016, 43, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Giatti, S.; Cioffi, L.; Diviccaro, S.; Piazza, R.; Melcangi, R.C. Analysis of the finasteride treatment and its withdrawal in the rat hypothalamus and hippocampus at whole-transcriptome level. J. Endocrinol. Investig. 2024, 47, 2565–2574. [Google Scholar] [CrossRef]
- Giatti, S.; Cioffi, L.; Diviccaro, S.; Chrostek, G.; Piazza, R.; Melcangi, R.C. Transcriptomic profile of the male rat hypothalamus and nucleus accumbes after paroxetine treatment and withdrawal: Possible causes of sexual dysfunction. Mol. Neurob. 2025, 62, 4935–4951. [Google Scholar] [CrossRef] [PubMed]
- Bolamperti, S.; Villa, I.; Rubinacci, A. Bone remodeling: An operational process ensuring survival and bone mechanical competence. Bone Res. 2022, 10, 48. [Google Scholar] [CrossRef]
- Duval, K.; Grover, H.; Han, L.-H.; Mou, Y.; Pegoraro, A.F.; Fredberg, J.; Chen, Z. Modeling Physiological Events in 2D vs. 3D Cell Culture. Physiology 2017, 32, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Kilian, K.A. Bridging the gap: From 2D cell culture to 3D microengineered extracellular matrices. Adv. Healthc. Mater. 2015, 4, 2780–2796. [Google Scholar] [CrossRef]
- Selim, M.; Mousa, H.M.; Abdel-Jaber, G.T.; Barhoum, A.; Abdal-hay, A. Innovative designs of 3D scaffolds for bone tissue regeneration: Understanding principles and addressing challenges. Eur. Polym. J. 2024, 215, 113251. [Google Scholar] [CrossRef]
- Nikolova, M.P.; Chavali, M.S. Recent advances in biomaterials for 3D scaffolds: A review. Bioact. Mater. 2019, 4, 271–292. [Google Scholar] [CrossRef]
- Steller, D.; Scheibert, A.; Sturmheit, T.; Rose, D.; Hakim, S.G. Impact of PRP and PRF on Viability of Zoledronic Acid Treated Osteoblasts in 2D and 3D Cell Culture. Anticancer Res. 2022, 42, 1295–1299. [Google Scholar] [CrossRef]
- Nune, K.C.; Kumar, A.; Misra, R.D.K.; Li, S.J.; Hao, Y.L.; Yang, R. Functional response of osteoblasts in functionally gradient titanium alloy mesh arrays processed by 3D additive manufacturing. Colloids Surf. B Biointerfaces 2017, 150, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Reznikov, N.; Shahar, R.; Weiner, S. Bone hierarchical structure in three dimensions. Acta Biomater. 2014, 10, 3815–3826. [Google Scholar] [CrossRef]
- Reznikov, N.; Bilton, M.; Lari, L.; Stevens, M.M.; Kröger, R. Fractal-like hierarchical organization of bone begins at the nanoscale. Science 2018, 360, eaao2189. [Google Scholar] [CrossRef]
- Yuste, I.; Luciano, F.C.; Gonzales-Burgos, E.; Lalatsa, A.; Serrano, D.R. Mimicking bone microenvironment: 2D and 3D in vitro models of human osteoblasts. Pharmacol. Res. 2021, 169, 105626. [Google Scholar] [CrossRef]
- Rumiński, S.; Ostrowska, B.; Jaroszewicz, J.; Skirecki, T.; Włodarski, K.; Święszkowski, W.; Lewandowska-Szumieł, M. Three-dimensional printed polycaprolactone-based scaffolds provide an advantageous environment for osteogenic differentiation of human adipose-derived stem cells. J. Tissue Eng. Regen. Med. 2018, 12, e473–e485. [Google Scholar] [CrossRef]
- Nune, K.C.; Kumar, A.; Misra, R.D.K.; Li, S.J.; Hao, Y.L.; Yang, R. Osteoblast functions in functionally graded Ti-6AI-4 V mesh structures. J. Biomater. Appl. 2016, 30, 1182–1204. [Google Scholar] [CrossRef]
- Nasello, G.; Alamán-Díez, P.; Schiavi, J.; Pérez, M.A.; McNamara, L.; García-Aznar, J.M. Primary Human Osteoblasts Cultured in a 3D Microenvironment Create a Unique Representative Model of Their Differentiation Into Osteocytes. Front. Bioeng. Biotechnol. 2020, 8, 336. [Google Scholar] [CrossRef]
- Gao, P.; Zhang, H.; Liu, Y.; Fan, B.; Li, X.; Xiao, X.; Lan, P.; Li, M.; Geng, L.; Liu, D.; et al. Beta-tricalcium phosphate granules improve osteogenesis in vitro and establish innovative osteo-regenerators for bone tissue engineering in vivo. Sci. Rep. 2016, 6, 23367. [Google Scholar] [CrossRef]
- Ohnishi, Y.; Yasui, H.; Kakudo, K.; Nozaki, M. Regulation of cell migration via the EGFR signaling pathway in oral squamous cell carcinoma cells. Oncol. Lett. 2017, 13, 930–936. [Google Scholar] [CrossRef]
- Mangiavini, L.; Peretti, G.M.; Canciani, B.; Maffulli, N. Epidermal growth factor signalling pathway in endochondral ossification: An evidence-based narrative review. Ann. Med. 2022, 54, 37–50. [Google Scholar] [CrossRef]
- Linder, M.; Hecking, M.; Glitzner, E.; Zwerina, K.; Holcmann, M.; Bakiri, L.; Ruocco, M.G.; Tuckermann, J.; Schett, G.; Wagner, E.F.; et al. EGFR controls bone development by negatively regulating mTOR-signaling during osteoblast differentiation. Cell Death Differ. 2018, 25, 1094–1106. [Google Scholar] [CrossRef] [PubMed]
- Chandra, A.; Lan, S.; Zhu, J.; Siclari, V.A.; Qin, L. Epidermal Growth Factor Receptor (EGFR) Signaling Promotes Proliferation and Survival in Osteoprogenitors by Increasing Early Growth Response 2 (EGR2) Expression. J. Biol. Chem. 2013, 288, 20488–20498. [Google Scholar] [CrossRef]
- Ventre, M.; Netti, P.A. Engineering Cell Instructive Materials To Control Cell Fate and Functions through Material Cues and Surface Patterning. ACS Appl. Mater. Interfaces 2016, 8, 14896–14908. [Google Scholar] [CrossRef]
- Rougerie, P.; Silva dos Santos, R.; Farina, M.; Anselme, K. Molecular Mechanisms of Topography Sensing by Osteoblasts: An Update. Appl. Sci. 2021, 11, 1791. [Google Scholar] [CrossRef]
- Uynuk-Ool, T.; Rothdiener, M.; Walters, B.; Hegemann, M.; Palm, J.; Nguyen, P.; Seeger, T.; Stöckle, U.; Stegemann, J.P.; Aicher, W.K.; et al. The geometrical shape of mesenchymal stromal cells measured by quantitative shape descriptors is determined by the stiffness of the biomaterial and by cyclic tensile forces. J. Tissue Eng. Regen. Med. 2017, 11, 3508–3522. [Google Scholar] [CrossRef] [PubMed]
- Castro-Raucci, L.M.S.; Francischini, M.S.; Teixeira, L.N.; Ferraz, E.P.; Lopes, H.B.; de Oliveira, P.T.; Hassan, M.Q.; Rosa, A.L.; Beloti, M.M. Titanium With Nanotopography Induces Osteoblast Differentiation by Regulating Endogenous Bone Morphogenetic Protein Expression and Signaling Pathway. J. Cell Biochem. 2016, 117, 1718–1726. [Google Scholar] [CrossRef]
- Abuna, R.P.F.; Oliveira, F.S.; Lopes, H.B.; Freitas, G.P.; Fernandes, R.R.; Rosa, A.L.; Beloti, M.M. The Wnt/β-catenin signaling pathway is regulated by titanium with nanotopography to induce osteoblast differentiation. Colloids Surf. B Biointerfaces 2019, 184, 110513. [Google Scholar] [CrossRef]
- Su, N.; Jin, M.; Chen, L. Role of FGF/FGFR signaling in skeletal development and homeostasis: Learning from mouse models. Bone Res. 2014, 2, 14003. [Google Scholar] [CrossRef]
- Xie, Y.; Su, N.; Yang, J.; Tan, Q.; Huang, S.; Jin, M.; Ni, Z.; Zhang, B.; Zhang, D.; Luo, F.; et al. FGF/FGFR signaling in health and disease. Signal Transduct. Target. Ther. 2020, 5, 181. [Google Scholar] [CrossRef]
- Ornitz, D.M.; Marie, P.J. Fibroblast growth factor signaling in skeletal development and disease. Genes Dev. 2015, 29, 1463–1486. [Google Scholar] [CrossRef]
- Charoenlarp, P.; Rajendran, A.K.; Iseki, S. Role of fibroblast growth factors in bone regeneration. Inflamm. Regen. 2017, 37, 10. [Google Scholar] [CrossRef] [PubMed]
- Fei, Y.; Xiao, L.; Doetschman, T.; Coffin, D.J.; Hurley, M.M. Fibroblast growth factor 2 stimulation of osteoblast differentiation and bone formation is mediated by modulation of the Wnt signaling pathway. J. Biol. Chem. 2011, 286, 40575–40583. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.S.; Kim, J.-H.; Singh, R.K.; Jang, J.-H.; Kim, H.-W. Therapeutic-designed electrospun bone scaffolds: Mesoporous bioactive nanocarriers in hollow fiber composites to sequentially deliver dual growth factors. Acta Biomater. 2015, 16, 103–116. [Google Scholar] [CrossRef]
- Negishi, T.; Mihara, N.; Chiba, T.; D’Armiento, J.; Chada, K.; Maeda, M.; Igarashi, M.; Imai, K. High mobility group AT-hook 2 regulates osteoblast differentiation and facial bone development. Biochem. Biophys. Res. Commun. 2022, 590, 68–74. [Google Scholar] [CrossRef]
- Zhao, H.; Yang, Y.; Wang, Y.; Feng, X.; Deng, A.; Ou, Z.; Chen, B. MicroRNA-497-5p stimulates osteoblast differentiation through HMGA2-mediated JNK signaling pathway. J. Orthop. Surg. Res. 2020, 15, 515. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Han, J.; Yang, X.; Wang, Y.; Pan, H.; Xu, L. Apoptosis repressor with caspase recruitment domain enhances survival and promotes osteogenic differentiation of human osteoblast cells under Zoledronate treatment. Mol. Med. Rep. 2016, 14, 3535–3542. [Google Scholar] [CrossRef]
- Hu, L.; Wang, Y.; Pan, H.; Kadir, K.; Wen, J.; Li, S.; Zhang, C. Apoptosis repressor with caspase recruitment domain (ARC) promotes bone regeneration of bone marrow-derived mesenchymal stem cells by activating Fgf-2/PI3K/Akt signaling. Stem Cell Res. Ther. 2021, 12, 185. [Google Scholar] [CrossRef]
- Catena, V.; Fanciulli, M. Deptor: Not only a mTOR inhibitor. J. Exp. Clin. Cancer Res. 2017, 36, 12. [Google Scholar] [CrossRef]
- Perez-Tejeiro, J.M.; Csukasi, F. DEPTOR in skeletal development and diseases. Front. Genet. 2021, 12, 667283. [Google Scholar] [CrossRef]
- Chen, S.; Jia, L.; Zhang, S.; Zheng, Y.; Zhou, Y. DEPTOR regulates osteogenic differentiation via inhibiting MEG3-mediated activation of BMP4 signaling and is involved in osteoporosis. Stem Cell Res. Ther. 2018, 9, 185. [Google Scholar] [CrossRef]
- Terruzzi, I.; Montesano, A.; Senesi, P.; Villa, I.; Ferraretto, A.; Bottani, M.; Vacante, F.; Spinello, A.; Bolamperti, S.; Luzi, L.; et al. L-Carnitine Reduces Oxidative Stress and Promotes Cells Differentiation and Bone Matrix Proteins Expression in Human Osteoblast-Like Cells. Biomed. Res. Int. 2019, 2019, 5678548. [Google Scholar] [CrossRef] [PubMed]
- Frattini, A.; Bolamperti, S.; Valli, R.; Cipolli, M.; Pinto, R.M.; Bergami, E.; Frau, M.R.; Cesaro, S.; Signo, M.; Bezzerri, V.; et al. Enhanced p53 Levels Are Involved in the Reduced Mineralization Capacity of Osteoblasts Derived from Shwachman–Diamond Syndrome Subjects. Int. J. Mol. Sci. 2021, 22, 13331. [Google Scholar] [CrossRef]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Danecek, P.; Bonfield, J.K.; Liddle, J.; Marshall, J.; Ohan, V.; Pollard, M.O.; Whitwham, A.; Keane, T.; McCarthy, S.A.; Davies, R.M.; et al. Twelve years of SAMtools and BCFtools. GigaScience 2021, 10, giab008. [Google Scholar] [CrossRef] [PubMed]
- Thorvaldsdóttir, H.; Robinson, J.T.; Jill, P.; Mesirov, J.P. Integrative Genomics Viewer (IGV): High-performance genomics data visualization and exploration. Brief. Bioinform. 2013, 14, 178–192. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castoldi, G.; Mauri, M.; D’Aliberti, D.; Spinelli, S.; Testa, L.; Gaverina, F.; Rubinacci, A.; Villa, I.; Bellelli, G.; Zerbini, G.; et al. Transcriptomic Profile of Human Osteoblast-like Cells Grown on Trabecular Titanium. Int. J. Mol. Sci. 2025, 26, 3598. https://doi.org/10.3390/ijms26083598
Castoldi G, Mauri M, D’Aliberti D, Spinelli S, Testa L, Gaverina F, Rubinacci A, Villa I, Bellelli G, Zerbini G, et al. Transcriptomic Profile of Human Osteoblast-like Cells Grown on Trabecular Titanium. International Journal of Molecular Sciences. 2025; 26(8):3598. https://doi.org/10.3390/ijms26083598
Chicago/Turabian StyleCastoldi, Giovanna, Mario Mauri, Deborah D’Aliberti, Silvia Spinelli, Leonardo Testa, Federico Gaverina, Alessandro Rubinacci, Isabella Villa, Giuseppe Bellelli, Gianpaolo Zerbini, and et al. 2025. "Transcriptomic Profile of Human Osteoblast-like Cells Grown on Trabecular Titanium" International Journal of Molecular Sciences 26, no. 8: 3598. https://doi.org/10.3390/ijms26083598
APA StyleCastoldi, G., Mauri, M., D’Aliberti, D., Spinelli, S., Testa, L., Gaverina, F., Rubinacci, A., Villa, I., Bellelli, G., Zerbini, G., Piazza, R., & Zatti, G. (2025). Transcriptomic Profile of Human Osteoblast-like Cells Grown on Trabecular Titanium. International Journal of Molecular Sciences, 26(8), 3598. https://doi.org/10.3390/ijms26083598






