Mechanistic Integration of Network Pharmacology and In Vivo Validation: TFRD Combat Osteoporosis via PI3K/AKT Pathway Activation
Abstract
:1. Introduction
2. Results
2.1. Results of Network Pharmacology Analysis
2.1.1. TFRD Component Target Prediction
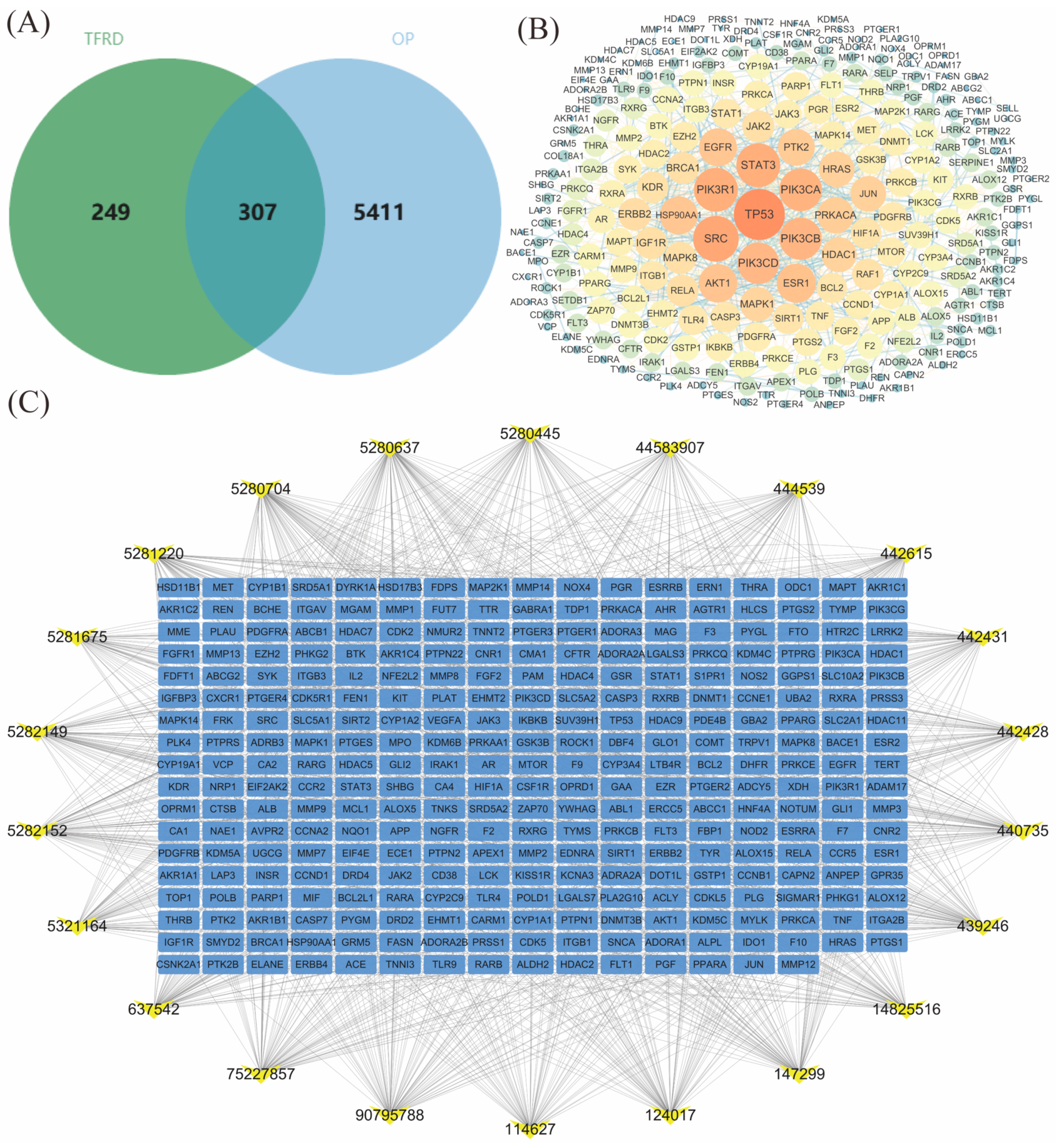
2.1.2. PPI Network Analysis of TFRD’s Common Action Targets in Treating OP
2.1.3. Construction of the “TFRD Component-Targets” Network
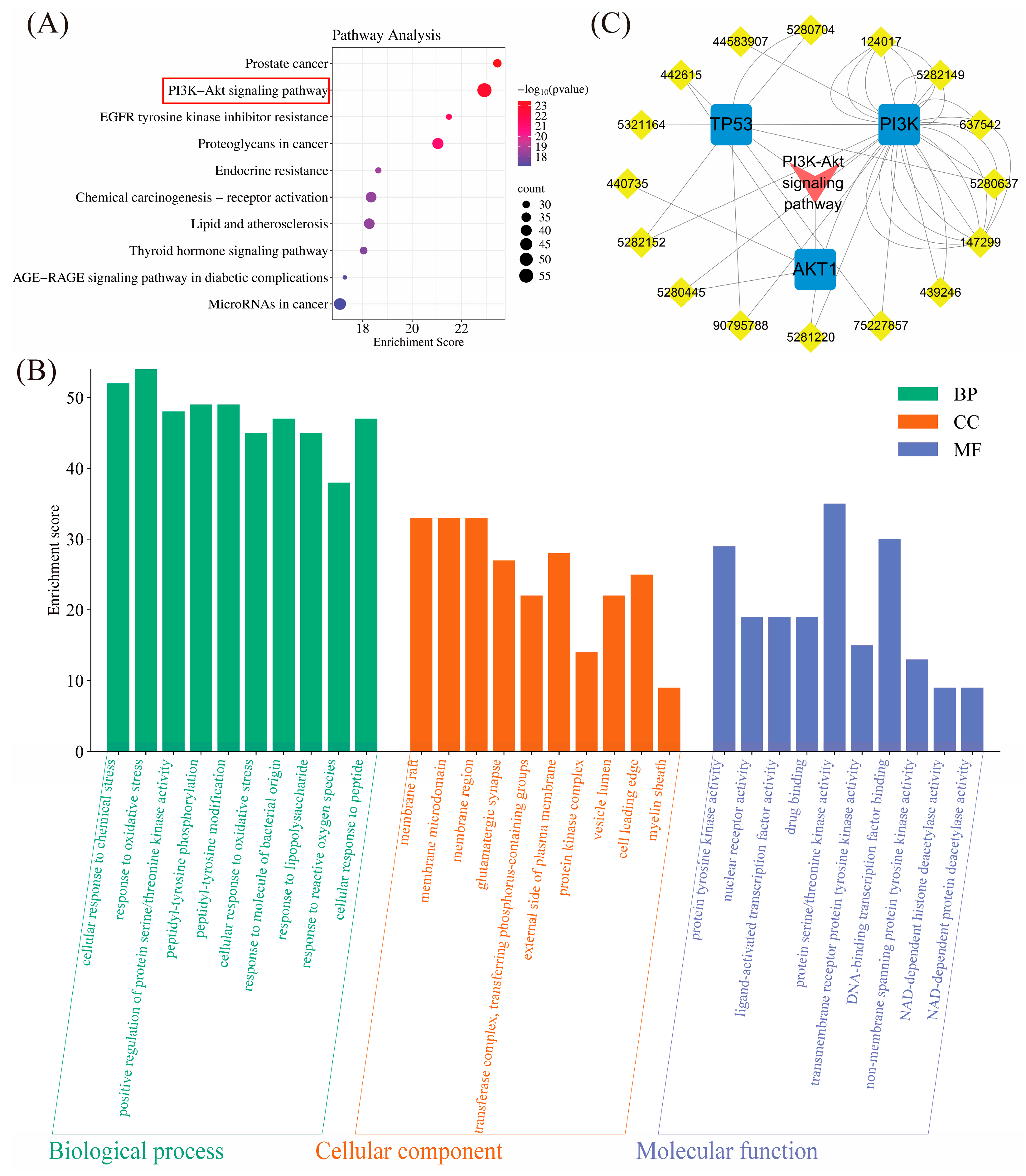
2.1.4. Pathway Enrichment Analysis
2.1.5. “TFRD Component-Target-Pathway” Network
2.2. In Vivo Experimental Results
2.2.1. Bone Morphometry and BMD Analysis
2.2.2. The Impact of TFRD on the BMD in Ovariectomized (OP) Rats
2.2.3. The Impact of TFRD on the BV/TV Ratio in Ovariectomized (OP) Rats
2.2.4. The Impact of TFRD on the Tb.Th Ratio in Ovariectomized (OP) Rats
2.2.5. The Impact of TFRD on the Tb.N Ratio in Ovariectomized (OP) Rats
2.2.6. The Impact of TFRD on the Tb.Sp Ratio in Ovariectomized (OP) Rats
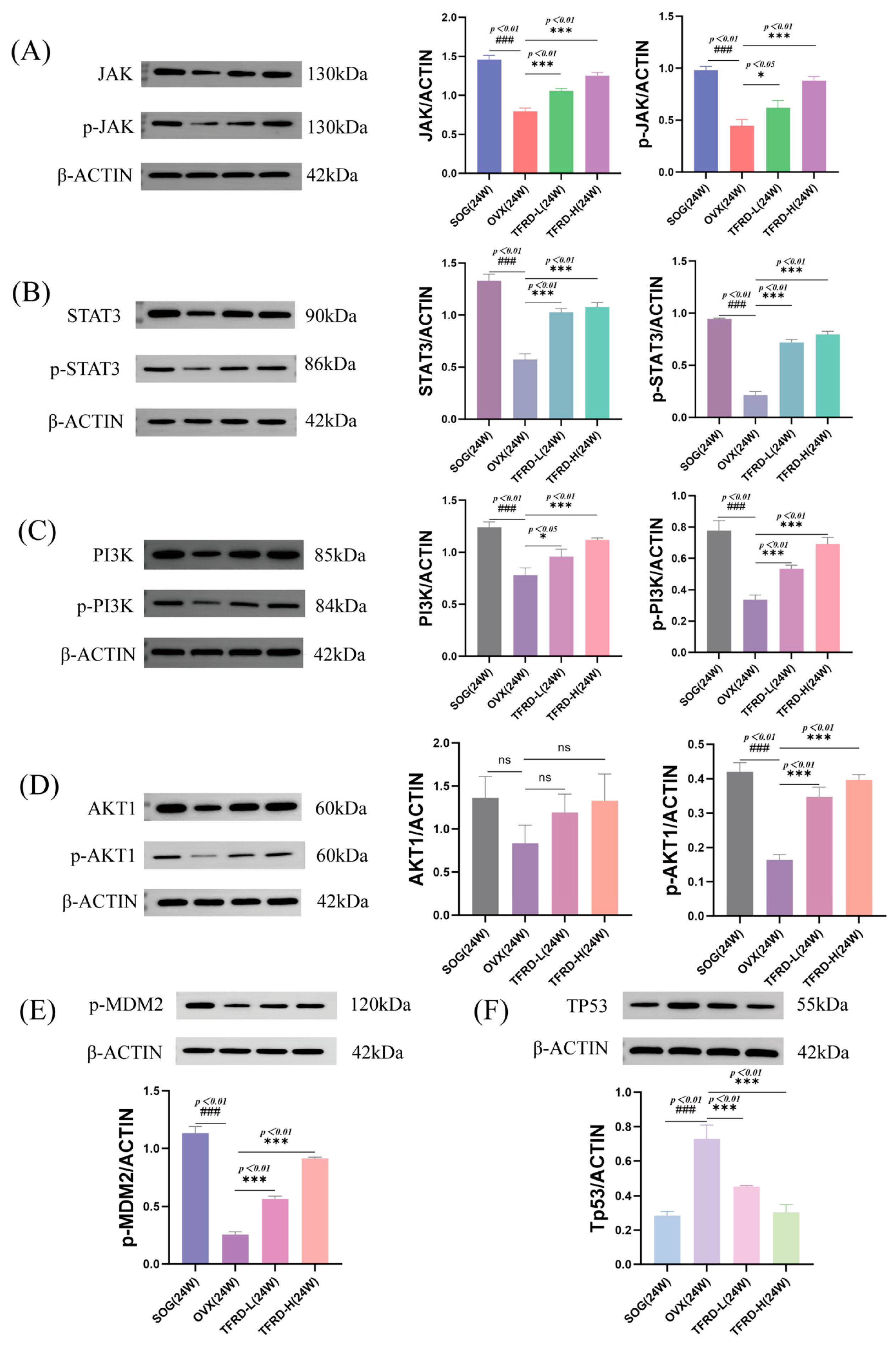
2.2.7. Detection of Protein Levels in the JAK/STAT3 and PI3K/AKT Pathways
2.2.8. Molecular Docking Verification
3. Discussion
4. Materials and Methods
4.1. Network Pharmacology Methods
4.1.1. Database and Tool Selection
4.1.2. Targets Prediction
4.1.3. PPI Network Construction
4.1.4. “TFRD Component-Targets” Network
4.1.5. Pathway Enrichment Analysis
4.1.6. “TFRD Component-Target-Pathway” Network
4.1.7. Grouping and Modeling of Experimental Animals
4.1.8. Drugs and Main Reagents
4.1.9. Detection Instruments for Bone Mineral Density (BMD) and Bone Morphological Indicators
4.1.10. H&E Staining Histological Analysis
4.1.11. Western Blot
4.1.12. Molecular Docking and Validation
4.1.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TFRD | The total flavonoids of Rhizoma Drynariae |
| OVX | Ovariectomized |
| TCM | Traditional Chinese medicine |
| PI3K | Phosphatidylinositol-3 kinase |
| AKT | Protein kinase B |
| BMD | Bone mineral density |
| OP | Osteoporosis |
| RANKL | Receptor activator of nuclear factor-κB ligand |
| E2 | Estradiol |
| CON | Control |
| SOG | Sham Operation Group |
| Sim | Positive drug |
| TFRD-L | Low-dose TFRD |
| TFRD-H | High-dose TFRD |
| PPI | Protein–protein interaction |
| BV | Bone volume |
| BV/TV | Bone volume fraction |
| Tb.Th | Trabecular thickness |
| Tb.Sp | Trabecular separation |
| Tb.N | Trabecular number |
References
- Ensrud, K.E.; Crandall, C.J. Osteoporosis. Ann. Intern. Med. 2024, 177, ITC1–ITC16. [Google Scholar] [CrossRef]
- Johnston, C.B.; Dagar, M. Osteoporosis in Older Adults. Med. Clin. N. Am. 2020, 104, 873–884. [Google Scholar] [CrossRef] [PubMed]
- Arceo-Mendoza, R.M.; Camacho, P.M. Postmenopausal Osteoporosis. Endocrinol. Metab. Clin. N. Am. 2021, 50, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, M.; Robinson, K.; Shibli-Rahhal, A. Bone Health and Osteoporosis Prevention and Treatment. Clin. Obstet. Gynecol. 2020, 63, 770–787. [Google Scholar] [CrossRef]
- Liu, F.; Wang, X.; He, Y.; Han, R.; Wang, T.; Guo, Y. Jaw Osteoporosis: Challenges to Oral Health and Emerging Perspectives of Treatment. Biomed. Pharmacother. 2024, 177, 116995. [Google Scholar] [CrossRef]
- Chen, Y.; Jia, L.; Han, T.; Zhao, Z.; Yang, J.; Xiao, J.; Yang, H.-J.; Yang, K. Osteoporosis Treatment: Current Drugs and Future Developments. Front. Pharmacol. 2024, 15, 1456796. [Google Scholar] [CrossRef]
- Aibar-Almazán, A.; Voltes-Martínez, A.; Castellote-Caballero, Y.; Afanador-Restrepo, D.F.; Carcelén-Fraile, M.D.C.; López-Ruiz, E. Current Status of the Diagnosis and Management of Osteoporosis. IJMS 2022, 23, 9465. [Google Scholar] [CrossRef] [PubMed]
- Eastell, R.; Rosen, C.J.; Black, D.M.; Cheung, A.M.; Murad, M.H.; Shoback, D. Pharmacological Management of Osteoporosis in Postmenopausal Women: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2019, 104, 1595–1622. [Google Scholar] [CrossRef]
- Cosman, F.; Crittenden, D.B.; Adachi, J.D.; Binkley, N.; Czerwinski, E.; Ferrari, S.; Hofbauer, L.C.; Lau, E.; Lewiecki, E.M.; Miyauchi, A.; et al. Romosozumab Treatment in Postmenopausal Women with Osteoporosis. N. Engl. J. Med. 2016, 375, 1532–1543. [Google Scholar] [CrossRef]
- Weaver, C.M.; Alexander, D.D.; Boushey, C.J.; Dawson-Hughes, B.; Lappe, J.M.; LeBoff, M.S.; Liu, S.; Looker, A.C.; Wallace, T.C.; Wang, D.D. Calcium plus Vitamin D Supplementation and Risk of Fractures: An Updated Meta-Analysis from the National Osteoporosis Foundation. Osteoporos. Int. 2016, 27, 367–376. [Google Scholar] [CrossRef]
- Gehrke, B.; Coelho, M.C.A.; d’Alva, C.B.; Madeira, M. Long-Term Consequences of Osteoporosis Therapy with Bisphosphonates. Arch. Endocrinol. Metab. 2023, 68, e220334. [Google Scholar] [CrossRef] [PubMed]
- Ayers, C.; Kansagara, D.; Lazur, B.; Fu, R.; Kwon, A.; Harrod, C. Effectiveness and Safety of Treatments to Prevent Fractures in People With Low Bone Mass or Primary Osteoporosis: A Living Systematic Review and Network Meta-Analysis for the American College of Physicians. Ann. Intern. Med. 2023, 176, 182–195. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Huang, X.; Qin, J.; Qi, B.; Sun, C.; Guo, X.; Liu, Q.; Liu, Y.; Ma, Y.; Wei, X.; et al. The Role of Traditional Chinese Medicines in the Treatment of Osteoporosis. Am. J. Chin. Med. 2024, 52, 949–986. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, J.; Shen, H.; Chai, Y.; Wei, X.; Xie, Y. Total Flavonoids from Rhizoma Drynariae (Gusuibu) for Treating Osteoporotic Fractures: Implication in Clinical Practice. DDDT 2017, 11, 1881–1890. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Jiang, N.; Xu, W.; Zhang, Z.; Yang, Y.; Zhang, J.; Xu, H. Effect of Flavonoids from Rhizoma Drynariae on Osteoporosis Rats and Osteocytes. Biomed. Pharmacother. 2022, 153, 113379. [Google Scholar] [CrossRef]
- Xu, T.; Zheng, J.; Jin, W.; Li, L.; Lin, L.; Shaukat, A.; Zhang, C.; Cao, Q.; Ashraf, M.; Huang, S. Corrigendum: Total Flavonoids of Rhizoma Drynariae Ameliorate Bone Growth in Experimentally Induced Tibial Dyschondroplasia in Chickens via Regulation of OPG/RANKL Axis. Front. Pharmacol. 2022, 13, 969027. [Google Scholar] [CrossRef]
- Lin, H.; Wang, X.; Li, Z.; Huang, M.; Feng, J.; Chen, H.; Gao, J.; Feng, Y.; Wu, J.; Tang, S.; et al. Total Flavonoids of Rhizoma Drynariae Promote Angiogenesis and Osteogenesis in Bone Defects. Phytother. Res. 2022, 36, 3584–3600. [Google Scholar] [CrossRef]
- Sun, K.; Luo, J.; Guo, J.; Yao, X.; Jing, X.; Guo, F. The PI3K/AKT/mTOR Signaling Pathway in Osteoarthritis: A Narrative Review. Osteoarthr. Cartil. 2020, 28, 400–409. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, Z.; Zhou, H.; Lin, S.; Gong, B.; Li, Z.; Zhao, S.; Hou, Y.; Peng, Y.; Bian, Y. Bazi Bushen Attenuates Osteoporosis in SAMP6 Mice by Regulating PI3K-AKT and Apoptosis Pathways. J. Cell. Mol. Med. 2024, 28, e70161. [Google Scholar] [CrossRef]
- Li, S.; Zhang, B. Traditional Chinese Medicine Network Pharmacology: Theory, Methodology and Application. Chin. J. Nat. Med. 2013, 11, 110–120. [Google Scholar] [CrossRef]
- Chai, S.; Yang, Y.; Wei, L.; Cao, Y.; Ma, J.; Zheng, X.; Teng, J.; Qin, N. Luteolin Rescues Postmenopausal Osteoporosis Elicited by OVX through Alleviating Osteoblast Pyroptosis via Activating PI3K-AKT Signaling. Phytomedicine 2024, 128, 155516. [Google Scholar] [CrossRef]
- Lin, Y.; Shi, R.; Wang, X.; Shen, H.-M. Luteolin, a Flavonoid with Potential for Cancer Prevention and Therapy. CCDT 2008, 8, 634–646. [Google Scholar] [CrossRef]
- Ersahin, T.; Tuncbag, N.; Cetin-Atalay, R. The PI3K/AKT/mTOR Interactive Pathway. Mol. BioSyst. 2015, 11, 1946–1954. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Liu, C.; Yu, X.; Gan, Z.; Xiang, L.; Zheng, J.; Meng, B.; Yu, R.; Chen, X.; et al. Mechanical Force-Activated CD109 on Periodontal Ligament Stem Cells Governs Osteogenesis and Osteoclast to Promote Alveolar Bone Remodeling. Stem. Cells Transl. Med. 2024, 13, 812–825. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Jiao, W.; Wu, D.-B.; Yu, J.-H.; Liu, L.-J.; Zhang, M.-Y.; Chen, G.-X. Yishen Tongbi Decoction Attenuates Inflammation and Bone Destruction in Rheumatoid Arthritis by Regulating JAK/STAT3/SOCS3 Pathway. Front. Immunol. 2024, 15, 1381802. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Dai, Q.; Huang, X.; Jin, A.; Yang, Y.; Gong, X.; Xu, H.; Gao, X.; Jiang, L. STAT3 Is Critical for Skeletal Development and Bone Homeostasis by Regulating Osteogenesis. Nat. Commun. 2021, 12, 6891. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. SwissTargetPrediction: Updated Data and New Features for Efficient Prediction of Protein Targets of Small Molecules. Nucleic Acids Res. 2019, 47, W357–W364. [Google Scholar] [CrossRef]
- Piñero, J.; Ramírez-Anguita, J.M.; Saüch-Pitarch, J.; Ronzano, F.; Centeno, E.; Sanz, F.; Furlong, L.I. The DisGeNET Knowledge Platform for Disease Genomics: 2019 Update. Nucleic Acids Res. 2019, 48, gkz1021. [Google Scholar] [CrossRef]
- Stelzer, G.; Rosen, N.; Plaschkes, I.; Zimmerman, S.; Twik, M.; Fishilevich, S.; Stein, T.I.; Nudel, R.; Lieder, I.; Mazor, Y.; et al. The GeneCards Suite: From Gene Data Mining to Disease Genome Sequence Analyses. CP Bioinform. 2016, 54, 1–30. [Google Scholar] [CrossRef]
- Amberger, J.S.; Bocchini, C.A.; Scott, A.F.; Hamosh, A. OMIM.Org: Leveraging Knowledge across Phenotype–Gene Relationships. Nucleic Acids Res. 2019, 47, D1038–D1043. [Google Scholar] [CrossRef]
- Davis, A.P.; Wiegers, T.C.; Sciaky, D.; Barkalow, F.; Strong, M.; Wyatt, B.; Wiegers, J.; McMorran, R.; Abrar, S.; Mattingly, C.J. Comparative Toxicogenomics Database’s 20th Anniversary: Update 2025. Nucleic Acids Res. 2025, 53, D1328–D1334. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhang, Y.; Zhao, D.; Yu, X.; Shen, X.; Zhou, Y.; Wang, S.; Qiu, Y.; Chen, Y.; Zhu, F. TTD: Therapeutic Target. Database Describing Target Druggability Information. Nucleic Acids Res. 2024, 52, D1465–D1477. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Furumichi, M.; Sato, Y.; Kawashima, M.; Ishiguro-Watanabe, M. KEGG for Taxonomy-Based Analysis of Pathways and Genomes. Nucleic Acids Res. 2023, 51, D587–D592. [Google Scholar] [CrossRef]
- Thomas, P.D.; Ebert, D.; Muruganujan, A.; Mushayahama, T.; Albou, L.; Mi, H. PANTHER: Making Genome-scale Phylogenetics Accessible to All. Protein Sci. 2022, 31, 8–22. [Google Scholar] [CrossRef]
- Eberhardt, J.; Santos-Martins, D.; Tillack, A.F.; Forli, S. AutoDock Vina 1.2.0: New Docking Methods, Expanded Force Field, and Python Bindings. J. Chem. Inf. Model. 2021, 61, 3891–3898. [Google Scholar] [CrossRef] [PubMed]

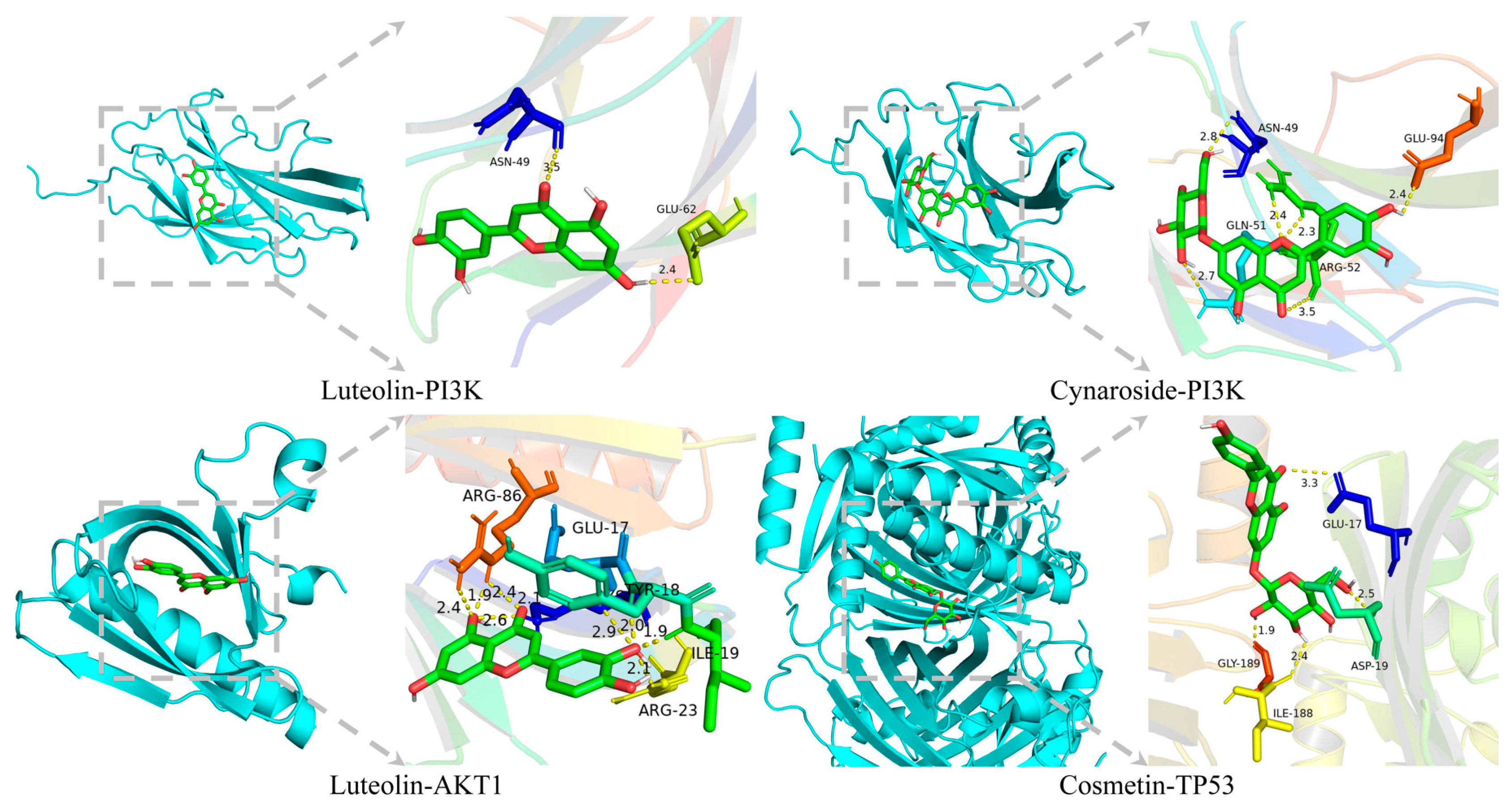
| PubChem CID | Component Name | Target | Binding Energy (kcal · mol−1) |
|---|---|---|---|
| 442,615 | Lucenin-2 | AKT1 | −6.2 |
| 440,735 | Eriodictyol | AKT1 | −6 |
| 5,280,445 | Luteolin | AKT1 | −6.3 |
| 5,281,220 | Aureusidin | PI3K | −5.8 |
| 439,246 | Naringenin | PI3K | −4.8 |
| 147,299 | Procyanidin B4 | PI3K | −5.5 |
| 637,542 | p-Coumaric acid | PI3K | −4.2 |
| 5,282,149 | Trifolin | PI3K | −5.5 |
| 124,017 | Procyanidin B5 | PI3K | −5.4 |
| 44,583,907 | 5-Hydroxy-7-(beta-D-glucopyranosyloxy)chromone | PI3K | −5.3 |
| 5,321,164 | Aureusidin-6-glucoside | PI3K | −4.9 |
| 5,282,152 | Lonicerin | PI3K | −5.2 |
| 90,795,788 | Kaempferol 3-O-Rhamnoside-7-O-Glucoside | PI3K | −3.9 |
| 75,227,857 | Kaempferol 3-O-beta-D-glucopyranoside-7-O-alpha-L-arabinofuranoside | PI3K | −4.9 |
| 5,280,637 | Cynaroside | PI3K | −6 |
| 5,280,445 | Luteolin | PI3K | −5.9 |
| 5,282,152 | Lonicerin | TP53 | −10.2 |
| 90,795,788 | Kaempferol 3-O-Rhamnoside-7-O-Glucoside | TP53 | −10.3 |
| 75,227,857 | Kaempferol 3-O-beta-D-glucopyranoside-7-O-alpha-L-arabinofuranoside | TP53 | −9.4 |
| 5,280,704 | Cosmetin | TP53 | −10.4 |
| 442,615 | Lucenin-2 | TP53 | −10.1 |
| 5,280,637 | Cynaroside | TP53 | −10.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, C.; Cong, S.; Xie, Y.; Zhi, Y. Mechanistic Integration of Network Pharmacology and In Vivo Validation: TFRD Combat Osteoporosis via PI3K/AKT Pathway Activation. Int. J. Mol. Sci. 2025, 26, 3650. https://doi.org/10.3390/ijms26083650
Tan C, Cong S, Xie Y, Zhi Y. Mechanistic Integration of Network Pharmacology and In Vivo Validation: TFRD Combat Osteoporosis via PI3K/AKT Pathway Activation. International Journal of Molecular Sciences. 2025; 26(8):3650. https://doi.org/10.3390/ijms26083650
Chicago/Turabian StyleTan, Chang, Shibo Cong, Yanming Xie, and Yingjie Zhi. 2025. "Mechanistic Integration of Network Pharmacology and In Vivo Validation: TFRD Combat Osteoporosis via PI3K/AKT Pathway Activation" International Journal of Molecular Sciences 26, no. 8: 3650. https://doi.org/10.3390/ijms26083650
APA StyleTan, C., Cong, S., Xie, Y., & Zhi, Y. (2025). Mechanistic Integration of Network Pharmacology and In Vivo Validation: TFRD Combat Osteoporosis via PI3K/AKT Pathway Activation. International Journal of Molecular Sciences, 26(8), 3650. https://doi.org/10.3390/ijms26083650






