Role of Regulatory T Cells in Pulmonary Ageing and COPD Development
Abstract
:1. Introduction
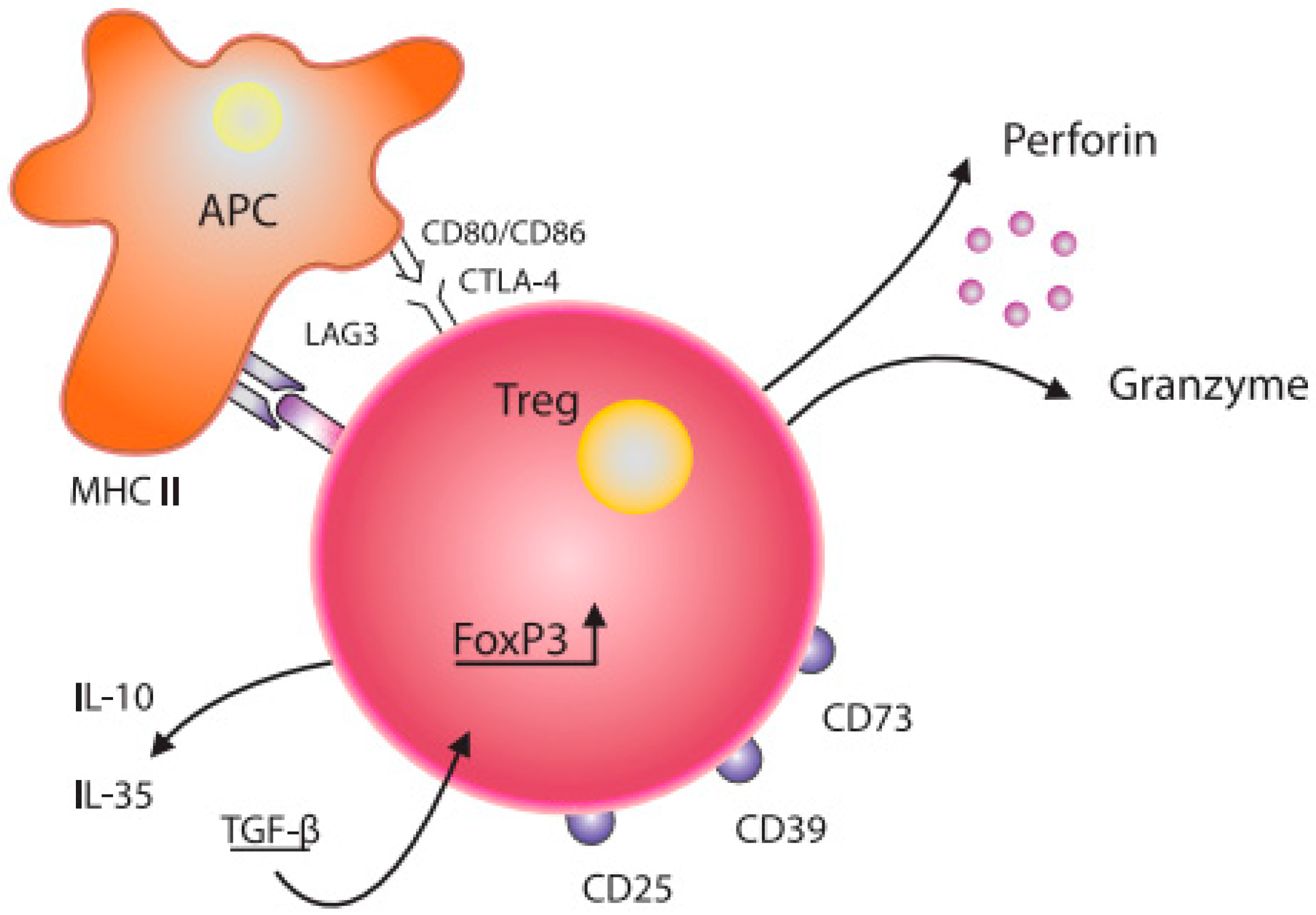
2. Treg Cells
3. Treg Cells and COPD
3.1. COPD Exhibits Features of Autoimmunity
3.2. A Distinct Phenotype of COPD May Involve Alternate Immunologic Mechanisms
3.3. Tregs in COPD Tissue
3.4. Metabolism and Immune Response in COPD
3.5. Granzyme B as an Effector Molecule and Potential Functional Marker for Treg Cells in COPD
3.6. Does the Level of Treg Cells Influence the Development of Lung Cancer?
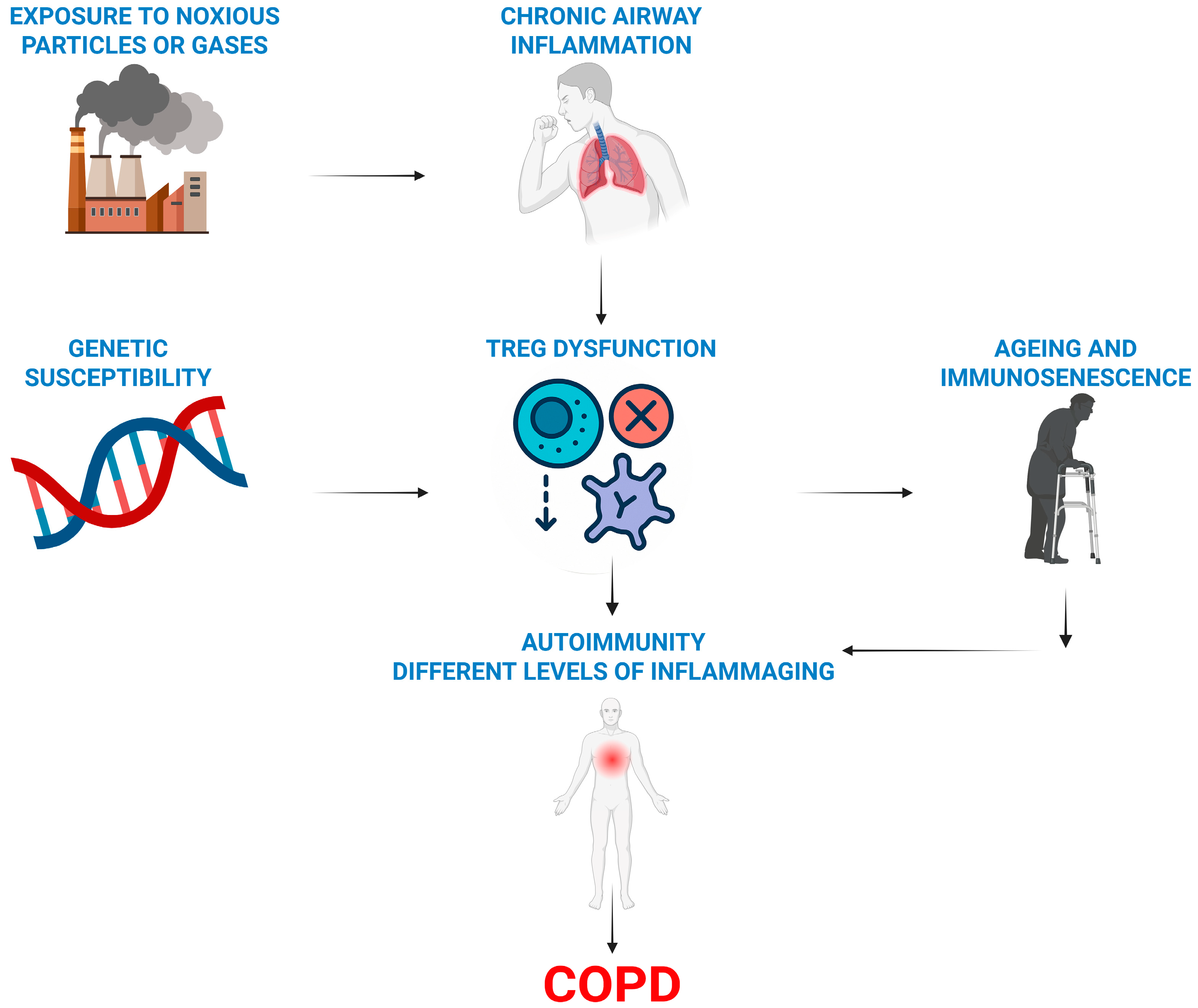
4. Treg Cells and Pulmonary Ageing
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Projections of Mortality and Causes of Death, 2016 to 2060; WHO: Geneva, Switzerland, 2018.
- Barnes, P.; Burney, P.; Silverman, E.; Celli, B.R.; Vestbo, J.; Wedzicha, J.A.; Wouters, E.F.M. Chronic obstructive pulmonary disease. Nat. Rev. Dis. Primers 2015, 1, 15076. [Google Scholar] [CrossRef] [PubMed]
- Szalontai, K.; Gémes, N.; Furák, J.; Varga, T.; Neuperger, P.; Balog, J.Á.; Puskás, L.G.; Szebeni, G.J. Chronic Obstructive Pulmonary Disease: Epidemiology, Biomarkers, and Paving the Way to Lung Cancer. J. Clin. Med. 2021, 10, 2889. [Google Scholar] [CrossRef] [PubMed]
- Agustí, A.; Faner, R. COPD beyond smoking: New paradigm, novel opportunities. Lancet Respir. Med. 2018, 6, 324–326. [Google Scholar] [CrossRef] [PubMed]
- Agusti, A.; Hogg, J.C. Update on the Pathogenesis of Chronic Obstructive Pulmonary Disease. N. Engl. J. Med. 2019, 381, 1248–1256. [Google Scholar] [CrossRef]
- Cosio, M.; Saetta, M.; Agusti, A. Immunological aspects of COPD. N. Engl. J. Med. 2009, 360, 2445–2454. [Google Scholar] [CrossRef]
- Hogg, J.C.; Chu, F.; Utokaparch, S.; Woods, R.; Elliott, W.M.; Buzatu, L.; Cherniack, R.M.; Rogers, R.M.; Sciurba, F.C.; Coxson, H.O.; et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N. Engl. J. Med. 2004, 350, 2645–2653. [Google Scholar] [CrossRef]
- Thomas, R.; Qiao, S.; Yang, X. Th17/Treg Imbalance: Implications in Lung Inflammatory Diseases. Int. J. Mol. Sci. 2023, 24, 4865. [Google Scholar] [CrossRef]
- Williams, L.; Rudensky, A. Maintenance of the Foxp3-dependent developmental program in mature regulatory T cells requires continued expression of Foxp3. Nat. Immunol. 2007, 8, 277–284. [Google Scholar] [CrossRef]
- Grossman, W.J.; Verbsky, J.W.; Tollefsen, B.L.; Kemper, C.; Atkinson, J.P.; Ley, T.J. Differential expression of granzymes a and b in human cytotoxic lymphocyte subsets and T regulatory cells. Blood 2004, 104, 2840–2848. [Google Scholar] [CrossRef]
- Cederbom, L.; Hall, H.; Ivars, F. CD4+CD25+ regulatory T cells down-regulate co-stimulatory molecules on antigen-presenting cells. Eur. J. Immunol. 2000, 30, 1538–1543. [Google Scholar] [CrossRef]
- Collison, L.W.; Workman, C.J.; Kuo, T.T.; Boyd, K.; Wang, Y.; Vignali, K.M.; Cross, R.; Sehy, D.; Blumberg, R.S.; Vignali, D.A.A. The inhibitory cytokine iL-35 contributes to regulatory T-cell function. Nature 2007, 450, 566–569. [Google Scholar] [CrossRef] [PubMed]
- Paterson, A.M.; Lovitch, S.B.; Sage, P.T.; Juneja, V.R.; Lee, Y.; Trombley, J.D.; Arancibia-Carcamo, C.V.; Sobel, R.A.; Rudensky, A.Y.; Kuchroo, V.K.; et al. Deletion of CTLA-4 on regulatory T cells during adulthood leads to resistance to autoimmunity. J. Exp. Med. 2015, 212, 1603–1621. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Hanabuchi, S.; Wang, Y.H.; Park, W.R.; Arima, K.; Bover, L.; Xiao-Feng Qin, F.; Gilliet, M.; Liu, Y.J. Two functional subsets of Foxp3+ regulatory T cells in human thymus and periphery. Immunity 2008, 28, 870–880. [Google Scholar] [CrossRef] [PubMed]
- Baecher-Allan, C.; Wolf, E.; Hafler, D.A. MHC class II expression identifies functionally distinct human regulatory T cells. J. Immunol. 2006, 176, 4622–4631. [Google Scholar] [CrossRef]
- Akkaya, B.; Oya, Y.; Akkaya, M.; Al Souz, J.; Holstein, A.H.; Kamenyeva, O.; Kabat, J.; Matsumura, R.; Dorward, D.W.; Glass, D.D.; et al. Regulatory T cells mediate specific suppression by depleting peptide-MHC class II from dendritic cells. Nat. Immunol. 2019, 20, 218–231. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Wing, J.B.; Sakaguchi, S. Two modes of immune suppression by Foxp3+ regulatory T cells under inflammatory or non-inflammatory conditions. Sem. Immunol. 2011, 23, 424–430. [Google Scholar] [CrossRef]
- Ye, J.; Huang, X.; Hsueh, E.C.; Zhang, Q.; Ma, C.; Zhang, Y.; Varvares, M.A.; Hoft, D.F.; Peng, G. Human regulatory T cells induce T-lymphocyte senescence. Blood 2012, 120, 2021–2031. [Google Scholar] [CrossRef]
- Birzele, F.; Fauti, T.; Stahl, H.; Lenter, M.C.; Simon, E.; Knebel, D.; Weith, A.; Hildebrandt, T.; Mennerich, D. Next-generation insights into regulatory T cells: Expression profiling and FoxP3 occupancy in human. Nucleic Acids Res. 2011, 39, 7946–7960. [Google Scholar] [CrossRef]
- Donnelly, C.; Dykstra, B.; Mondal, N.; Huang, J.; Kaskow, B.J.; Griffin, R.; Sackstein, R.; Baecher-Allan, C. Optimizing human treg immunotherapy by treg subset selection and e-selectin ligand expression. Sci. Rep. 2018, 8, 420. [Google Scholar] [CrossRef]
- Miyara, M.; Yoshioka, Y.; Kitoh, A.; Shima, T.; Wing, K.; Niwa, A.; Parizot, C.; Taflin, C.; Heike, T.; Valeyre, D.; et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the Foxp3 transcription factor. Immunity 2009, 30, 899–911. [Google Scholar] [CrossRef]
- Roncarolo, M.G.; Gregori, S. Is FOXP3 a bona fide marker for human regulatory T cells? Eur. J. Immunol. 2008, 38, 925–927. [Google Scholar] [CrossRef] [PubMed]
- Bailey-Bucktrout, S.L.; Martinez-Llordella, M.; Zhou, X.; Anthony, B.; Rosenthal, W.; Luche, H.; Fehling, H.F.; Bluestone, J.A. Self-antigen-driven activation induces instability of regulatory T cells during an inflammatory autoimmune response. Immunity 2013, 39, 949–962. [Google Scholar] [CrossRef] [PubMed]
- Shevach, E.M. Regulatory/suppressor T cells in health and disease. Arthr. Rheum. 2004, 50, 2721–2724. [Google Scholar] [CrossRef] [PubMed]
- Fessler, J.; Felber, A.; Duftner, C.; Dejaco, C. Therapeutic potential of regulatory T cells in autoimmune disorders. BioDrugs 2013, 27, 281–291. [Google Scholar] [CrossRef]
- Battaglia, M.; Roncarolo, M.G. The fate of human treg cells. Immunity 2009, 30, 763–765. [Google Scholar] [CrossRef]
- Saetta, M.; Baraldo, S.; Corbino, L.; Turato, G.; Braccioni, F.; Rea, F.; Cavallesco, G.; Tropeano, G.; Mapp, C.E.; Maestrelli, P.; et al. CD8+ve cells in the lungs of smokers with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 1999, 160, 711–717. [Google Scholar] [CrossRef]
- Kheradmand, F.; Shan, M.; Xu, C.; Corry, D.B. Autoimmunity in chronic obstructive pulmonary disease: Clinical and experimental evidence. Expert. Rev. Clin. Immunol. 2012, 8, 285–292. [Google Scholar] [CrossRef]
- Domagała-Kulawik, J.; Hoser, G.; Dąbrowska, M.; Safianowska, A.; Chazan, R. CD4+/CD25+ cells in systemic inflammation in COPD. Scand. J. Immunol. 2011, 73, 59–65. [Google Scholar] [CrossRef]
- Sileikiene, V.; Laurinaviciene, A.; Lesciute-Krilaviciene, D.; Jurgauskiene, L.; Malickaite, R.; Laurinavicius, A. Levels of CD4+ CD25+ T Regulatory Cells in Bronchial Mucosa and Peripheral Blood of Chronic Obstructive Pulmonary Disease Indicate Involvement of Autoimmunity Mechanisms. Adv. Respir. Med. 2019, 87, 159–166. [Google Scholar] [CrossRef]
- Chu, S.; Zhong, X.; Zhang, J.; Lao, Q.; He, Z.; Bai, J. The Expression of Foxp3 and ROR Gamma T in Lung Tissues From Normal Smokers and Chronic Obstructive Pulmonary Disease Patients. Int. Immunopharmacol. 2011, 11, 1780–1788. [Google Scholar] [CrossRef]
- Eriksson Ström, J.; Pourazar, J.; Linder, R.; Blomberg, A.; Lindberg, A.; Bucht, A.; Behndig, A. Airway Regulatory T Cells are Decreased in COPD with a Rapid Decline in Lung Function. Respir. Res. 2020, 21, 330. [Google Scholar] [CrossRef] [PubMed]
- Li, X.N.; Pan, X.; Qiu, D. Imbalances of Th17 and Treg cells and their respective cytokines in COPD patients by disease stage. Int. J. Clin. Exp. Med. 2014, 7, 5324–5329. [Google Scholar] [PubMed]
- Liu, W.; Putnam, A.L.; Xu-Yu, Z.; Szot, G.L.; Lee, M.R.; Zhu, S.; Gottlieb, P.A.; Kapranov, P.; Gingeras, T.R.; Fazekas de St Groth, B.; et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J. Exp. Med. 2006, 203, 1701–1711. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Huo, B.; Zhong, X.; Su, W.; Liu, W.; Li, Y.; He, Z.; Bai, J. Imbalance between Sub-populations of Regulatory T Cells in Patients with Acute Exacerbation of COPD. COPD J. Chronic Obstr. Pulm. Dis. 2017, 14, 618–625. [Google Scholar] [CrossRef]
- Chiappori, A.; Folli, C.; Balbi, F.; Caci, E.; Riccio, A.M.; Ferrari, L.; Melioli, G.; Braido, F.; Canonica, G.W. CD4(+)CD25(high)CD127(-) regulatory T-cells in COPD: Smoke and drugs effect. World Allergy Organ J. 2016, 9, 5. [Google Scholar] [CrossRef]
- Barceló, B.; Pons, J.; Ferrer, J.M.; Sauleda, J.; Fuster, A.; Agusti, A.G.N. Phenotypic characterisation of T-lymphocytes in COPD: Abnormal CD4+ CD25+ regulatory T-lymphocyte response to tobacco smoking. Eur. Respir. J. 2008, 31, 555–562. [Google Scholar] [CrossRef]
- Smyth, L.J.C.; Starkey, C.; Vestbo, J.; Singh, D. CD4-regulatory cells in COPD patients. Chest 2007, 132, 156–163. [Google Scholar] [CrossRef]
- Hou, J.; Wang, X.; Su, C.; Ma, W.; Zheng, X.; Ge, X.; Duan, X. Reduced frequencies of Foxp3+GARP+ regulatory T cells in COPD patients are associated with multi-organ loss of tissue phenotype. Respir. Res. 2022, 23, 176. [Google Scholar] [CrossRef]
- Cappello, F.; Caramori, G.; Campanella, C.; Vicari, C.; Gnemmi, I.; Zanini, A.; Spanevello, A.; Capelli, A.; La Rocca, G.; Anzalone, R.; et al. Convergent sets of data from in vivo and in vitro methods point to an active role of Hsp60 in chronic obstructive pulmonary disease pathogenesis. PLoS ONE 2011, 6, e28200. [Google Scholar] [CrossRef]
- Pridgeon, C.; Bugeon, L.; Donnelly, L.; Straschil, U.; Tudhope, S.J.; Fenwick, P.; Lamb, J.R.; Barnes, P.J.; Dallman, M.J. Regulation of IL-17 in chronic inflammation in the human lung. Clin. Sci. 2011, 120, 515–524. [Google Scholar] [CrossRef]
- Sales, D.S.; Ito, J.T.; Zanchetta, I.A.; Annoni, R.; Aun, M.V.; Ferraz, L.F.S.; Cervilha, D.A.B.; Negri, E.; Manad, T.; Martins, M.A.; et al. Regulatory T-Cell Distribution within Lung Compartments in COPD. COPD 2017, 14, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Isajevs, S.; Taivans, I.; Strazda, G.; Kopeika, U.; Bukovskis, M.; Gordjusina, V.; Kratovska, A. Decreased FOXP3 expression in small airways of smokers with COPD. Eur. Respir. J. 2009, 33, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Caramori, G.; Casolari, P.; Barczyk, A.; Durham, A.L.; Di Stefano, A.; Adcock, I. COPD immunopathology. Semin. Immunopathol. 2016, 38, 497–515. [Google Scholar] [CrossRef] [PubMed]
- Brusselle, G.G.; Joos, G.F.; Bracke, K.R. New insights into the immunology of chronic obstructive pulmonary disease. Lancet 2011, 378, 1015–1026. [Google Scholar] [CrossRef]
- Plumb, J.; Smyth, L.J.C.; Adams, H.R.; Vestbo, J.; Bentley, A.; Singh, S.D. Increased T-regulatory cells within lymphocyte follicles in moderate COPD. Eur. Respir. J. 2009, 34, 89–94. [Google Scholar] [CrossRef]
- Caramori, G.; Ruggeri, P.; Di Stefano, A.; Mumby, S.; Girbino, G.; Adcock, I.; Kirkham, P. Autoimmunity and COPD: Clinical Implications. Chest 2018, 153, 1424–1431. [Google Scholar] [CrossRef]
- D’Alessio, F.R.; Tsushima, K.; Aggarwal, N.R.; West, E.E.; Willett, M.H.; Britos, M.F.; Pipeling, M.R.; Brower, R.G.; Tuder, R.M.; McDyer, J.F.; et al. CD4+ CD25+Foxp3+ Tregs resolve experimental lung injury in mice and are present in humans with acute lung injury. J. Clin. Investig. 2009, 119, 2898–2913. [Google Scholar] [CrossRef]
- Lourenco, J.D.; Ito, J.T.; Martins, M.A.; Tiberio, I.F.L.C.; Lopes, F.D.T.Q.S. Th17/Treg Imbalance in Chronic Obstructive Pulmonary Disease: Clinical and Experimental Evidence. Front. Immunol. 2021, 12, 804919. [Google Scholar] [CrossRef]
- Lee, S.H.; Goswami, S.; Grudo, A.; Song, L.Z.; Bandi, V.; Goodnight-White, S.; Green, L.; Hacken-Bitar, J.; Huh, J.; Bakaeen, F.; et al. Antielastin Autoimmunity in Tobacco Smoking-Induced Emphysema. Nat. Med. 2007, 13, 567–569. [Google Scholar] [CrossRef]
- Lourenço, J.D.; Teodoro, W.R.; Barbeiro, D.F.; Velosa, A.P.P.; Silva, L.E.F.; Kohler, J.B.; Moreira, A.R.; Aun, M.V.; da Silva, I.C.; Fernandes, F.L.A.; et al. Th17/Treg-Related Intracellular Signaling in Patients with Chronic Obstructive Pulmonary Disease: Comparison Between Local and Systemic Responses. Cells 2021, 10, 1569. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, L.; Chen, J.; Gu, Y.; Xu, J.; Ouyang, Y. Dendritic Cells and Th17/Treg Ratio Play Critical Roles in Pathogenic Process of Chronic Obstructive Pulmonary Disease. BioMed Pharmacother. 2018, 108, 1141–1151. [Google Scholar] [CrossRef] [PubMed]
- Freeman, C.M.; McCubbrey, A.L.; Crudgington, S.; Nelson, J.; Martinez, F.J.; Han, M.K.; Washko, G.R., Jr.; Chensue, S.W.; Arenberg, D.A.; Meldrum, C.A.; et al. Basal gene expression by lung CD4+ T cells in chronic obstructive pulmonary disease identifies independent molecular correlates of airflow obstruction and emphysema extent. PLoS ONE 2014, 9, e96421. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, H.; Ying, H.; Wang, S.; Gu, X.; Weng, Y.; Peng, W.; Xia, D.; Yu, W. Imbalance of Peripheral Blood Th17 and Treg Responses in Patients with Chronic Obstructive Pulmonary Disease. Clin. Respir. J. 2015, 9, 330–341. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Rojas, M.I.; Ramırez-Venegas, A.; Limon-Camacho, L.; Ochoa, L.; Hernaández-Zenteno, R.; Sansores, R.H. Increase of Th17 Cells in Peripheral Blood of Patients with Chronic Obstructive Pulmonary Disease. Respir. Med. 2011, 105, 1648–1654. [Google Scholar] [CrossRef]
- Bruzzaniti, S.; Bocchino, M.; Santopaolo, M.; Cali, G.; Stanziola, A.A.; D’Amato, M.; Esposito, A.; Barra, E.; Garziano, F.; Micillo, T.; et al. An immunometabolic pathomechanism for chronic obstructive pulmonary disease. Proc. Natl. Acad. Sci. USA 2019, 116, 15625–15634. [Google Scholar] [CrossRef]
- Gondek, C.D.; Lu, L.-F.; Quezada, S.A.; Sakaguchi, S.; Noelle, R.J. Cutting Edge: Contact-Mediated Suppression by CD4+CD25+ Regulatory Cells Involves a Granzyme B-Dependent, Perforin-Independent Mechanism. J. Immunol. 2005, 174, 1783–1786. [Google Scholar] [CrossRef]
- Kim, W.D.; Sin, D.D. Granzyme B May Act as an Effector Molecule to Control the Inflammatory Process in COPD. COPD J. Chronic Obstr. Pulm. Dis. 2024, 21, 2299104. [Google Scholar] [CrossRef]
- Wauters, E.; Janssens, W.; Vansteenkiste, J.; Decaluwé, H.; Heulens, N.; Thienpont, B.; Zhao, H.; Smeets, D.; Sagaert, X.; Coolen, J.; et al. DNA methylation profilling of non-small-cell lung cancer reveals a COPD-driven immune-related signature. Thorax 2015, 70, 1113–1122. [Google Scholar] [CrossRef]
- Balestro, E.; Baraldo, S.; Piloni, D.; Stella, G.M. Lung tumors, COPD and immune response: Is epigenetics the bottom line? Minerva Med. 2016, 107 (Suppl. 1), 1–8. [Google Scholar]
- Faner, R.; Rojas, M.; Macnee, W.; Agusti, A. Abnormal lung aging in chronic obstructive pulmonary disease and idiopathic pulmonary fibrosis. Am. J. Resp. Crit. Care Med. 2012, 186, 306–313. [Google Scholar] [CrossRef]
- Cho, W.K.; Lee, C.G.; Kim, L.K. COPD as a disease of immunosenescence. Yonsei Med. J. 2019, 60, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Goronzy, J.J.; Weyand, C.M. Immune aging and autoimmunity. Cell Mol. Life Sci. 2012, 69, 1615–1623. [Google Scholar] [CrossRef] [PubMed]
- Berzins, S.P.; Uldrich, A.P.; Sutherland, J.S.; Gill, J.; Miller, J.F.; Godfrey, D.I.; Boyd, R.L. Thymic regeneration: Teaching an old immune system new tricks. Trends Mol. Med. 2002, 8, 469–476. [Google Scholar] [CrossRef] [PubMed]
- van der Geest, K.S.; Abdulahad, W.H.; Tete, S.M.; Lorencetti, P.G.; Horst, G.; Bos, N.A.; Kroesen, B.J.; Brouwer, E.; Boots, A.M.H. Aging disturbs the balance between effector and regulatory CD4+ T cells. Exp. Gerontol. 2014, 60, 190–196. [Google Scholar] [CrossRef]
- Simone, R.; Zicca, A.; Saverino, D. The frequency of regulatory CD3+CD8+CD28−CD25+ T lymphocytes in human peripheral blood increases with age. J. Leukocyte Biol. 2008, 84, 1454–1461. [Google Scholar] [CrossRef]
- Hou, J.; Sun, Y. Role of Regulatory T Cells in Disturbed Immune Homeostasis in Patients with Chronic Obstructive Pulmonary Disease. Front. Immunol. 2020, 11, 723. [Google Scholar] [CrossRef]
- Ito, K.; Barnes, P.J. COPD as a disease of accelerated lung aging. CHEST J. 2009, 135, 173–180. [Google Scholar] [CrossRef]
- Ghadially, R.; Brown, B.E.; Sequeira-Martin, S.M.; Feingold, K.R.; Elias, P.M. The aged epidermal permeability barrier. Structural, functional, and lipid biochemical abnormalities in humans and a senescent murine model. J. Clin. Investig. 1995, 95, 2281–2290. [Google Scholar] [CrossRef]
- Ho, J.C.; Chan, K.N.; Hu, W.H.; Lam, W.K.; Zheng, L.; Tipoe, G.L.; Sun, J.; Leung, R.; Tsang, K.W. The effect of aging on nasal mucociliary clearance, beat frequency, and ultrastructure of respiratory cilia. Am. J. Respir. Crit. Care Med. 2001, 163, 983–988. [Google Scholar] [CrossRef]
- Shugars, D.C.; Watkins, C.A.; Cowen, H.J. Salivary concentration of secretory leukocyte protease inhibitor, an antimicrobial protein, is decreased with advanced age. Gerontology 2001, 47, 246–253. [Google Scholar] [CrossRef]
- Tsuji, T.; Aoshiba, K.; Nagai, A. Alveolar cell senescence in patients with pulmonary emphysema. Am. J. Respir. Crit. Care Med. 2006, 174, 886–893. [Google Scholar] [CrossRef]
- Kirkland, J.L.; Tchkonia, T. Cellular senescence: A translational perspective. EBioMedicine 2017, 21, 21–28. [Google Scholar] [CrossRef]
| Pulmonary Response | Systemic Response | |
|---|---|---|
| Treg | ↓ lung tissue of COPD (II-IV) vs. control [50] ↑ lung tissue of COPD (I-II) vs. healthy smokers [51] ↓ BALF of COPD (II-III) vs. healthy smokers [37] ↓ lung tissue of COPD (II-III) vs. healthy smokers and control [31,52] ↓ bronchial epithelium of COPD (III-IV) vs. COPD (I-II) [30] ↓ BALF of COPD (rapid decline in lung function) vs. COPD (nonrapid decline) [32] ↓ small airways of COPD (I-IIII) vs. control [42,43] ↑ large airways of COPD (II) vs. control [43] ↑ BALF of COPD (I-III) and healthy smokers vs. control [38] ↑ lymphoid tissue of COPD (I-III) vs. control [42,46] ↓lung tissue of COPD vs. healthy smokers [53] | ↓ peripheral blood of COPD (II-III) vs. healthy smokers and control [54] ↓ peripheral blood of COPD (I-II) vs. healthy smokers [51] ↑ peripheral blood of COPD (II-IV) vs. control [55] ↓ peripheral blood of COPD (III-IV) vs. COPD (I-II) [30] ↓ peripheral blood of exacerbated COPD vs. stable COPD [33] ↓ peripheral blood of exacerbated COPD vs. stable COPD, healthy smokers, and control [52] ↓ peripheral blood of COPD (I-II) vs. healthy smokers and control [29] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šileikienė, V.; Jurgauskienė, L. Role of Regulatory T Cells in Pulmonary Ageing and COPD Development. Int. J. Mol. Sci. 2025, 26, 3721. https://doi.org/10.3390/ijms26083721
Šileikienė V, Jurgauskienė L. Role of Regulatory T Cells in Pulmonary Ageing and COPD Development. International Journal of Molecular Sciences. 2025; 26(8):3721. https://doi.org/10.3390/ijms26083721
Chicago/Turabian StyleŠileikienė, Virginija, and Laimutė Jurgauskienė. 2025. "Role of Regulatory T Cells in Pulmonary Ageing and COPD Development" International Journal of Molecular Sciences 26, no. 8: 3721. https://doi.org/10.3390/ijms26083721
APA StyleŠileikienė, V., & Jurgauskienė, L. (2025). Role of Regulatory T Cells in Pulmonary Ageing and COPD Development. International Journal of Molecular Sciences, 26(8), 3721. https://doi.org/10.3390/ijms26083721






