Optimization and Bioreactor Scale-Up of Cellulase Production in Trichoderma sp. KMF006 for Higher Yield and Performance
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effect of Carbon Source Composition on Cellulase Activity
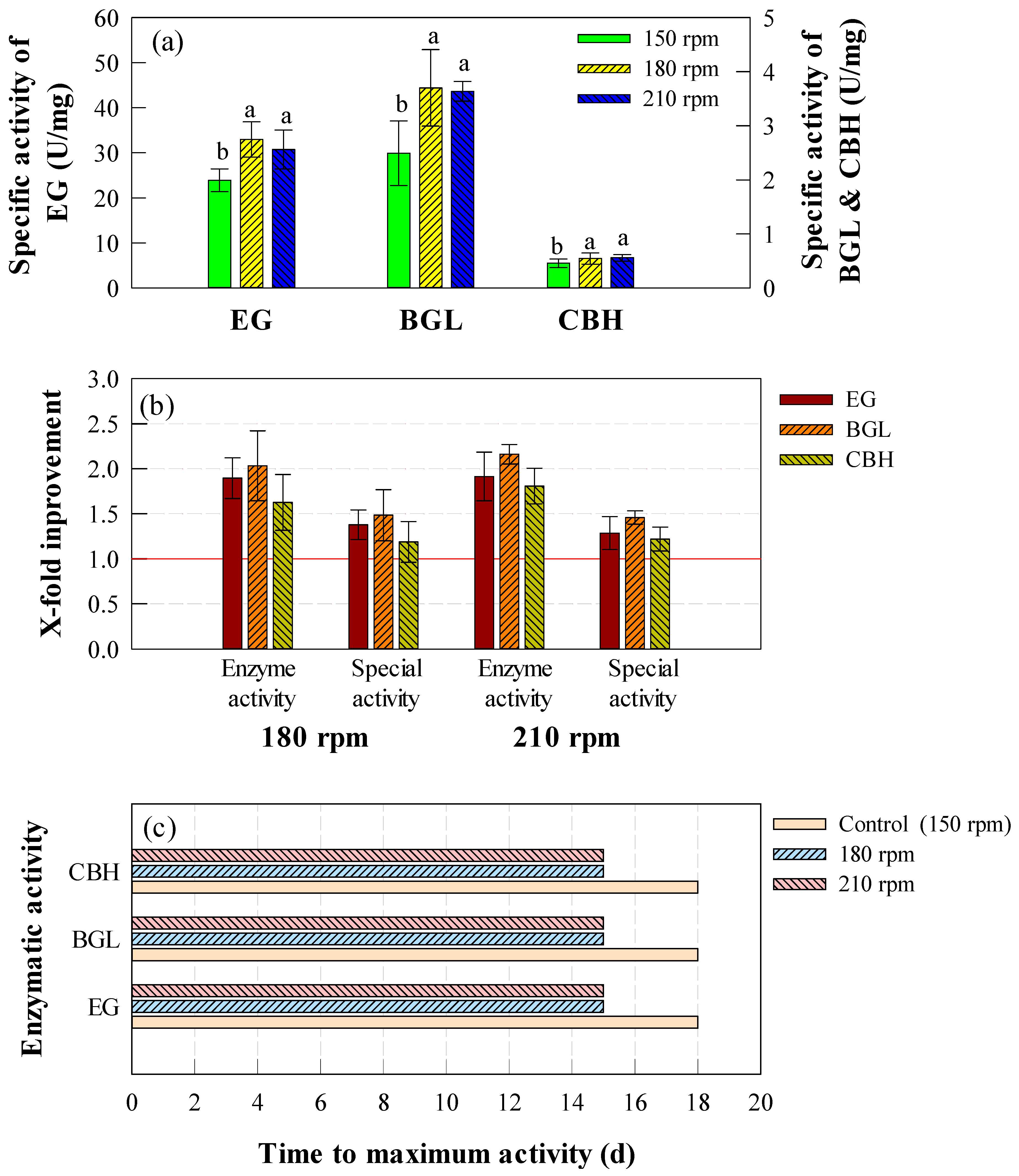
2.2. Effect of Agitation Speed on Cellulase Activity
2.3. Effect of Turbulence on Cellulase Activity
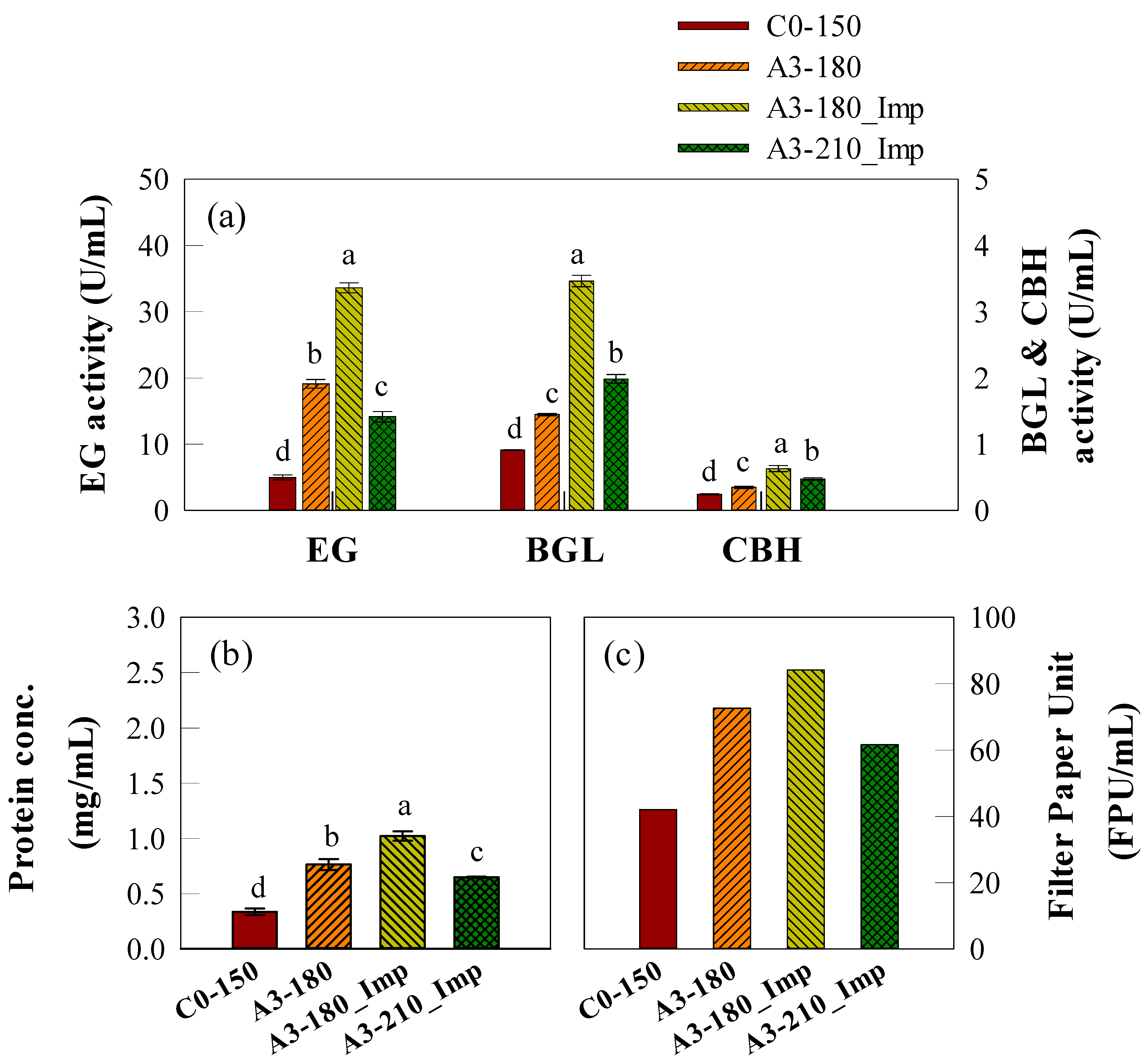
2.4. Scale-Up of Cellulase Production in a SmF Bioreactor
3. Materials and Methods
3.1. Microorganism and Pre-Culture Conditions
3.2. Assessment of Carbon Source Composition on Cellulase Production
3.3. Assessment of Agitation Speed on Cellulase Production
3.4. Assessment of Turbulence Efficiency on Cellulase Production
3.5. SmF Bioreactor Conditions for High Activity Cellulase Production
3.6. Enzymatic Activity and Protein Analysis
3.6.1. Enzymatic Activity Analysis
3.6.2. Protein Concentration Analysis
3.6.3. Filter Paper Unit (FPU) Analysis
3.6.4. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Razzak, S.A.; Hossain, M.M.; Lucky, R.A.; Bassi, A.S.; De Lasa, H. Integrated CO2 capture, wastewater treatment and biofuel production by microalgae culturing—A review. Renew. Sust. Energy Rev. 2013, 27, 622–653. [Google Scholar] [CrossRef]
- Ibrahim, N.I.; Shahar, F.S.; Hameed Sultan, M.T.; Md Shah, A.U.; Azrie Safri, S.N.; Mat Yazik, M.H. Overview of bioplastic introduction and its applications in product packaging. Coatings 2021, 11, 1423. [Google Scholar] [CrossRef]
- Rodionova, M.V.; Bozieva, A.M.; Zharmukhamedov, S.K.; Leong, Y.K.; Chi-Wei Lan, J.; Veziroglu, A.; Veziroglu, T.N.; Tomo, T.; Chang, J.S.; Allakhverdiev, S.I. A comprehensive review on lignocellulosic biomass biorefinery for sustainable biofuel production. Int. J. Hydrogen Energy 2022, 47, 1481–1498. [Google Scholar] [CrossRef]
- Mangal, M.; Rao, C.V.; Banerjee, T. Bioplastic: An eco-friendly alternative to non-biodegradable plastic. Polym. Int. 2023, 72, 984–996. [Google Scholar] [CrossRef]
- Kudakasseril Kurian, J.; Raveendran Nair, G.; Hussain, A.; Vijaya Raghavan, G.S. Feedstocks, logistics and pre-treatment processes for sustainable lignocellulosic biorefineries: A comprehensive review. Renew. Sust. Energy Rev. 2013, 25, 205–219. [Google Scholar] [CrossRef]
- Rodionova, M.V.; Poudyal, R.S.; Tiwari, I.; Voloshin, R.A.; Zharmukhamedov, S.K.; Nam, H.G.; Zayadan, B.K.; Bruce, B.D.; Hou, H.J.M.; Allakhverdiev, S.I. Biofuel production: Challenges and opportunities. Int. J. Hydrogen Energy 2017, 42, 8450–8461. [Google Scholar] [CrossRef]
- Yu, D.; Guo, J.; Meng, J.; Sun, T. Biofuel production by hydro-thermal liquefaction of municipal solid waste: Process characterization and optimization. Chemosphere 2023, 328, 138606. [Google Scholar] [CrossRef]
- Shah, M.; Rajhans, M.; Pandya, H.A.; Mankad, A.U. Mankad bioplastic for future: A review Then and Now. World J. Adv. Res. Rev. 2021, 9, 056–067. [Google Scholar] [CrossRef]
- Siqueira, J.G.W.; Rodrigues, C.; Vandenberghe, L.P.d.S.; Woiciechowski, A.L.; Soccol, C.R. Current advances in on-site cellulase production and application on lignocellulosic biomass conversion to biofuels: A review. Biomass Bioenergy 2020, 132, 105419. [Google Scholar] [CrossRef]
- REN21 Secretariat. Renewable 2018 Global Status Report; REN21: Paris, France, 2018; Available online: https://www.ren21.net/wp-content/uploads/2019/08/Full-Report-2018.pdf (accessed on 10 March 2025).
- Eckert, S.; Karassin, O.; Steinebach, Y. A policy portfolio approach to plastics throughout their life cycle: Supranational and national regulation in the European Union. Environ. Policy Gov. 2024, 34, 427–441. [Google Scholar] [CrossRef]
- Van Dyk, J.S.; Pletschke, B.I. A review of lignocellulose bioconversion using enzymatic hydrolysis and synergistic cooperation between enzymes—Factors affecting enzymes, conversion and synergy. Biotechnol. Adv. 2012, 30, 1458–1480. [Google Scholar] [CrossRef]
- Woo, W.X.; Tan, J.P.; Wu, T.Y.; Yeap, S.K.; Luthfi, A.A.I.; Manaf, S.F.A.; Jamali, N.S.; Hui, Y.W. An overview on the factors affecting enzymatic saccharification of lignocellulosic biomass into fermentable sugars. Rev. Chem. Eng. 2024, 40, 279–303. [Google Scholar] [CrossRef]
- Abo, B.O.; Gao, M.; Wang, Y.; Wu, C.; Ma, H.; Wang, Q. Lignocellulosic biomass for bioethanol: An overview on pretreatment, hydrolysis and fermentation processes. Rev. Environ. Health 2019, 34, 57–68. [Google Scholar] [CrossRef]
- El-Zawawy, W.K.; Ibrahim, M.M.; Abdel-Fattah, Y.R.; Soliman, N.A.; Mahmoud, M.M. Acid and enzyme hydrolysis to convert pretreated lignocellulosic materials into glucose for ethanol production. Carbohydr. Polym. 2011, 84, 865–871. [Google Scholar] [CrossRef]
- Sukumaran, R.K.; Singhania, R.R.; Mathew, G.M.; Pandey, A. Cellulase production using biomass feed stock and its application in lignocellulose saccharification for bio-ethanol production. Renew. Energy 2009, 34, 421–424. [Google Scholar] [CrossRef]
- Singh, A.; Bajar, S.; Devi, A.; Pant, D. An overview on the recent developments in fungal cellulase production and their industrial applications. Bioresour. Technol. Rep. 2021, 14, 100652. [Google Scholar] [CrossRef]
- Klein-Marcuschamer, D.; Oleskowicz-Popiel, P.; Simmons, B.A.; Blanch, H.W. The challenge of enzyme cost in the production of lignocellulosic biofuels. Biotechnol. Bioeng. 2012, 109, 1083–1087. [Google Scholar] [CrossRef]
- Singh, A.; Bajar, S.; Devi, A.; Bishnoi, N.R. Evaluation of cellulase production from Aspergillus niger and Aspergillus heteromorphus under submerged and solid-state fermentation. Environ. Sustain. 2021, 4, 437–442. [Google Scholar] [CrossRef]
- Korsa, G.; Konwarh, R.; Masi, C.; Ayele, A.; Haile, S. Microbial cellulase production and its potential application for textile industries. Ann. Microbiol. 2023, 73, 13. [Google Scholar] [CrossRef]
- Srivastava, N.; Srivastava, M.; Mishra, P.K.; Singh, P.; Ramteke, P.W. Application of cellulases in biofuels industries: An overview. J. Biofuels Bioenergy 2015, 1, 55–63. [Google Scholar] [CrossRef]
- Infanzón-Rodríguez, M.I.; Ragazzo-Sánchez, J.A.; del Moral, S.; Calderón-Santoyo, M.; Gutiérrez-Rivera, B.; Aguilar-Uscanga, M.G. Optimization of cellulase production by Aspergillus niger ITV 02 from sweet sorghum bagasse in submerged culture using a Box–Behnken design. Sugar Tech. 2020, 22, 266–273. [Google Scholar] [CrossRef]
- Matkar, K.; Chapla, D.; Divecha, J.; Nighojkar, A.; Madamwar, D. Production of cellulase by a newly isolated strain of Aspergillus sydowii and its optimization under submerged fermentation. Int. Biodeterior. Biodegrad. 2013, 78, 24–33. [Google Scholar] [CrossRef]
- Ramanathan, G.; Banupriya, S.; Abirami, D. Production and optimization of cellulase from Fusarium oxysporum by submerged fermentation. Bioprocess. Biosyst. Eng. 2010, 69, 454–459. [Google Scholar]
- Reihani, S.F.S.; Khosravi-Darani, K. Influencing factors on single-cell protein production by submerged fermentation: A review. Electron. J. Biotechnol. 2019, 37, 34–40. [Google Scholar] [CrossRef]
- Cunha, F.M.; Esperança, M.N.; Zangirolami, T.C.; Badino, A.C.; Farinas, C.S. Sequential solid-state and submerged cultivation of Aspergillus niger on sugarcane bagasse for the production of cellulase. Bioresour. Technol. 2012, 112, 270–274. [Google Scholar] [CrossRef]
- Intasit, R.; Cheirsilp, B.; Suyotha, W.; Boonsawang, P. Synergistic production of highly active enzymatic cocktails from lignocellulosic palm wastes by sequential solid-state submerged fermentation and co-cultivation of different filamentous fungi. Biochem. Eng. J. 2021, 173, 108086. [Google Scholar] [CrossRef]
- da Silva Delabona, P.; Lima, D.J.; Robl, D.; Rabelo, S.C.; Farinas, C.S.; da Cruz Pradella, J.G. Enhanced cellulase production by Trichoderma harzianum by cultivation on glycerol followed by induction on cellulosic substrates. J. Ind. Microbiol. Biotechnol. 2016, 43, 617–626. [Google Scholar] [CrossRef]
- Lan, T.Q.; Wei, D.; Yang, S.T.; Liu, X. Enhanced cellulase production by Trichoderma viride in a rotating fibrous bed bioreactor. Bioresour. Technol. 2013, 133, 175–182. [Google Scholar] [CrossRef]
- Myeong, S.; Yun, J. Culture of Trichoderma sp. with biochar to produce high-activity cellulase in a laboratory. Bioresources 2024, 19, 2029–2044. [Google Scholar] [CrossRef]
- Du, J.; Zhang, Y.; Qu, M.; Yin, Y.; Fan, K.; Hu, B.; Zhang, H.; Wei, M.; Ma, C. Effects of biochar on the microbial activity and community structure during sewage sludge composting. Bioresour. Technol. 2019, 272, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Awad, Y.M.; Lee, S.S.; Kim, K.H.; Ok, Y.S.; Kuzyakov, Y. Carbon and nitrogen mineralization and enzyme activities in soil aggregate-size classes: Effects of biochar, oyster shells, and polymers. Chemosphere 2018, 198, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Li, M.; Tao, X.; Yang, C.; Zhang, W.; Li, H.; Zheng, Y.; Wang, X.; Chen, R. Biochar enhanced organic matter transformation during pig manure composting: Roles of the cellulase activity and fungal community. J. Environ. Manag. 2023, 333, 117464. [Google Scholar] [CrossRef]
- Mohanty, S.K.; Valenca, R.; Berger, A.W.; Yu, I.K.M.; Xiong, X.; Saunders, T.M.; Tsang, D.C.W. Plenty of room for carbon on the ground: Potential applications of biochar for stormwater treatment. Sci. Total Environ. 2018, 625, 1644–1658. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Preparation, modification and environmental application of biochar: A review. J. Clean. Prod. 2019, 227, 1002–1022. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Feng, Y. The effects of biochar addition on soil physicochemical properties: A review. Catena 2021, 202, 105284. [Google Scholar] [CrossRef]
- Kim, Y.; Park, S.; Kim, Y. Novel Trichoderma sp. KMF006 Strain Producing Cellulase with High Activity. Korean Patent No. 10-2155046, 11 September 2020. [Google Scholar]
- Kogo, T.; Yoshida, Y.; Koganei, K.; Matsumoto, H.; Watanabe, T.; Ogihara, J.; Kasumi, T. Production of rice straw hydrolysis enzymes by the fungi Trichoderma reesei and Humicola insolens using rice straw as a carbon source. Bioresour. Technol. 2017, 233, 67–73. [Google Scholar] [CrossRef]
- Silva, J.C.R.; Salgado, J.C.S.; Vici, A.C.; Ward, R.J.; Polizeli, M.L.T.M.; Guimarães, L.H.S.; Furriel, R.P.M.; Jorge, J.A. A novel Trichoderma reesei mutant RP698 with enhanced cellulase production. Braz. J. Microbiol. 2020, 51, 537–545. [Google Scholar] [CrossRef]
- Hodrová, B.; Kopečný, J.; Káš, J. Cellulolytic enzymes of rumen anaerobic fungi Orpinomyces joyonii and Caecomyces communis. Res. Microbiol. 1998, 149, 417–427. [Google Scholar] [CrossRef]
- Karnchanatat, A.; Petsom, A.; Sangvanich, P.; Piapukiew, J.; Whalley, A.J.S.; Reynolds, C.D.; Gadd, G.M.; Sihanonth, P. A novel thermostable endoglucanase from the wood-decaying fungus Daldinia eschscholzii (Ehrenb.:Fr.) Rehm. Enzyme Microb. Technol. 2008, 42, 404–413. [Google Scholar] [CrossRef]
- Singhania, R.R.; Saini, R.; Adsul, M.; Saini, J.K.; Mathur, A.; Tuli, D. An integrative process for bio-ethanol production employing SSF produced cellulase without extraction. Biochem. Eng. J. 2015, 102, 45–48. [Google Scholar] [CrossRef]
- Saini, R.; Saini, J.K.; Adsul, M.; Patel, A.K.; Mathur, A.; Tuli, D.; Singhania, R.R. Enhanced cellulase production by Penicillium oxalicum for bio-ethanol application. Bioresour. Technol. 2015, 188, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Qian, Y.; Wang, Y.; Qu, Y.; Zhong, Y. Production of the versatile cellulase for cellulose bioconversion and cellulase inducer synthesis by genetic improvement of Trichoderma reesei. Biotechnol. Biofuels 2017, 10, 272. [Google Scholar] [CrossRef]
- Li, Y.; Liu, C.; Bai, F.; Zhao, X. Overproduction of cellulase by Trichoderma reesei RUT C30 through batch-feeding of synthesized low-cost sugar mixture. Bioresour. Technol. 2016, 216, 503–510. [Google Scholar] [CrossRef]
- Suto, M.; Tomita, F. Induction and catabolite repression mechanisms of cellulase in fungi. J. Biosci. Bioeng. 2001, 92, 305–308. [Google Scholar] [CrossRef]
- Kubicek, C.P.; Mikus, M.; Schuster, A.; Schmoll, M.; Seiboth, B. Metabolic engineering strategies for the improvement of cellulase production by Hypocrea jecorina. Biotechnol. Biofuels 2009, 2, 19. [Google Scholar] [CrossRef]
- Yu, L.; Chao, Y.; Wensel, P.; Chen, S. Hydrodynamic and kinetic study of cellulase production by Trichoderma reesei with pellet morphology. Biotechnol. Bioeng. 2012, 109, 1755–1768. [Google Scholar] [CrossRef]
- Sirohi, R.; Singh, A.; Tarafdar, A.; Shahi, N.C.; Verma, A.K.; Kushwaha, A. Cellulase production from pre-treated pea hulls using Trichoderma reesei under submerged fermentation. Waste Biomass Valorization 2019, 10, 2651–2659. [Google Scholar] [CrossRef]
- Teoh, Y.; Don, M.M.; Fadzilah, K. Optimization of cellulase production by Pycnoporus sanguineus in 5 L stirred tank bioreactor and enhanced fermentation by employing external loop. Chiang Mai J. Sci. 2017, 44, 774–787. [Google Scholar]
- Mmango-Kaseke, Z.; Okaiyeto, K.; Nwodo, U.U.; Mabinya, L.V.; Okoh, A.I. Optimization of cellulase and xylanase production by Micrococcus species under submerged fermentation. Sustainability 2016, 8, 1168. [Google Scholar] [CrossRef]
- Park, J.K.; Hyun, S.H.; Jung, J.Y. Conversion of G. hansenii PJK into non-cellulose-producing mutants according to the culture condition. Biotechnol. Bioprocess. Eng. 2004, 9, 383–388. [Google Scholar] [CrossRef]
- Mitra, S.; Banerjee, P.; Gachhui, R.; Mukherjee, J. Cellulase and xylanase activity in relation to biofilm formation by two intertidal filamentous fungi in a novel polymethylmethacrylate conico-cylindrical flask. Bioprocess. Biosyst. Eng. 2011, 34, 1087–1101. [Google Scholar] [CrossRef]
- Batra, J.; Beri, D.; Mishra, S. Response surface methodology-based optimization of β-glucosidase production from Pichia pastoris. Appl. Biochem. Biotechnol. 2014, 172, 380–393. [Google Scholar] [CrossRef]
- Weber, J.; Agblevor, F.A. Microbubble fermentation of Trichoderma reesei for cellulase production. Process Biochem. 2005, 40, 669–676. [Google Scholar] [CrossRef]
- Ma, H.; Liu, Y.; Li, Z. The synergistic hydrogen production of bicellular fermentation systems and fluid dynamics simulation in reactor under stirring. Bioresour. Technol. Rep. 2023, 22, 101473. [Google Scholar] [CrossRef]
- Dey, P.; Rangarajan, V.; Singh, J.; Nayak, J.; Dilip, K.J. Current perspective on improved fermentative production and purification of fungal cellulases for successful biorefinery applications: A brief review. Biomass Convers. Biorefin. 2022, 12, 967–995. [Google Scholar] [CrossRef]
- Shokrkar, H.; Ebrahimi, S.; Zamani, M. A review of bioreactor technology used for enzymatic hydrolysis of cellulosic materials. Cellulose 2018, 25, 6279–6304. [Google Scholar] [CrossRef]
- Chisti, Y.; Jauregui-Haza, U.J. Oxygen transfer and mixing in mechanically agitated airlift bioreactors. Bioprocess. Eng. 2002, 10, 143–153. [Google Scholar] [CrossRef]
- de Albuquerque de Carvalho, M.L.; Carvalho, D.F.; de Barros Gomes, E.; Maeda, R.N.; Anna, L.M.M.S.; de Castro, A.M.; Pereira, N., Jr. Optimisation of cellulase production by Penicillium funiculosum in a stirred tank bioreactor using multivariate response surface analysis. Enzyme Res. 2014, 2014, 703291. [Google Scholar] [CrossRef]
- Kim, S.W.; Kang, S.W.; Lee, J.S. Cellulase and xylanase production by Aspergillus niger KKS in various bioreactors. Bioresour. Technol. 1997, 59, 63–67. [Google Scholar] [CrossRef]
- Ahamed, A.; Vermette, P. Culture-based strategies to enhance cellulase enzyme production from Trichoderma reesei RUT-C30 in bioreactor culture conditions. Biochem. Eng. J. 2008, 40, 399–407. [Google Scholar] [CrossRef]
- Lejeune, R.; Baron, G.V. Effect of agitation on growth and enzyme production of Trichoderma reesei in batch fermentation. Biotechnol. Bioeng. 1995, 43, 249–258. [Google Scholar] [CrossRef]
- Hayward, T.K.; Hamilton, J.; Tholudur, A.; McMillan, J.D. Improvements in titer, productivity, and yield using Solka-Floc for cellulase production. In Proceedings of the Twenty-First Symposium on Biotechnology for Fuels and Chemicals, Fort Collins, CO, USA, 2–6 May 1999; Humana Press: Totowa, NJ, USA, 2000; pp. 859–874. [Google Scholar]
- Nelson, N. A photometric adaptation of the Somogyi method for the determination of glucose. J. Biol. Chem. 1944, 153, 375–380. [Google Scholar] [CrossRef]
- Joo, A.R.; Jeya, M.; Lee, K.M.; Sim, W.I.; Kim, J.S.; Kim, I.W.; Kim, Y.S.; Oh, D.K.; Gunasekaran, P.; Lee, J.K. Purification and characterization of a β-1,4-glucosidase from a newly isolated strain of Fomitopsis pinicola. Appl. Microbiol. Biotechnol. 2009, 83, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- NREL. Measurement of cellulase activities: Laboratory analytical procedure (LAP). In NREL Technical Report (NREL/TP-510-42628); 2008. Available online: https://www.nrel.gov/docs/gen/fy08/42628.pdf (accessed on 12 April 2025).


| C Source Comp. (1) | EG (U/mL) | BGL (U/mL) | CBH (U/mL) | Protein (mg/mL) |
| Control (A4C0) | 24.19 ± 5.18 ab | 0.80 ± 0.05 ab | 0.20 ± 0.01 b | 0.58 ± 0.09 a |
| A3C1 | 27.84 ± 3.29 ab | 0.87 ± 0.14 a | 0.25 ± 0.01 a | 0.68 ± 0.19 a |
| A2C2 | 17.93 ± 5.55 b | 0.72 ± 0.05 ab | 0.18 ± 0.03 b | 0.73 ± 0.06 a |
| A1C3 | 16.75 ± 3.13 b | 0.70 ± 0.07 b | 0.17 ± 0.02 b | 0.60 ± 0.07 a |
| A0C4 | 21.38 ± 4.88 ab | 0.66 ± 0.05 b | 0.20 ± 0.01 b | 0.66 ± 0.06 a |
| Agitation speed (2) | EG (U/mL) | BGL (U/mL) | CBH (U/mL) | Protein (mg/mL) |
| 150 rpm | 16.90 ± 2.12 b | 1.77 ± 0.42 b | 0.33 ± 0.06 b | 0.71 ± 0.08 b |
| 180 rpm | 32.04 ± 3.82 a | 3.60 ± 0.69 a | 0.53 ± 0.10 a | 1.05 ± 0.07 a |
| 210 rpm | 32.35 ± 4.57 a | 3.83 ± 0.19 a | 0.59 ± 0.06 a | 1.10 ± 0.05 a |
| Turbulence effi. (3) | EG (U/mL) | BGL (U/mL) | CBH (U/mL) | Protein (mg/mL) |
| Baf-w/o BC | 27.59 ± 3.03 b | 1.15 ± 0.07 b | 0.30 ± 0.01 b | 0.69 ± 0.04 b |
| Baf-1% BC | 34.41 ± 3.02 a | 1.46 ± 0.15 a | 0.33 ± 0.02 a | 0.97 ± 0.03 a |
| Rou-w/o BC | 10.18 ± 1.14 c | 0.63 ± 0.09 c | 0.14 ± 0.02 c | 0.43 ± 0.05 c |
| Rou-1% BC | 1.73 ± 1.05 d | 0.24 ± 0.02 d | 0.04 ± 0.00 d | 0.42 ± 0.07 c |
| Scale | Operational parameter | Experiment | Experimental Condition |
|---|---|---|---|
| Flask | C source composition (1) | A4C0 (Control) | Avicel:Cellulose = 4:0 (w/v), 150 rpm |
| A3C1 | Avicel:Cellulose = 3:1 (w/v), 150 rpm | ||
| A2C2 | Avicel:Cellulose = 2:2 (w/v), 150 rpm | ||
| A1C3 | Avicel:Cellulose = 1:3 (w/v), 150 rpm | ||
| A0C4 | Avicel:Cellulose = 0:4 (w/v), 150 rpm | ||
| Agitation speed (2) | 150 rpm | Fixed C source ratio (A3C1), 150 rpm | |
| 180 rpm | Fixed C source ratio (A3C1), 180 rpm | ||
| 210 rpm | Fixed C source ratio (A3C1), 210 rpm | ||
| Turbulence efficiency (3) | Baf-w/o BC | Fixed C source ratio (A3C1), 180 rpm, baffled flask, no biochar | |
| Baf-1% BC | Fixed C source ratio (A3C1), 180 rpm, baffled flask, 1% biochar | ||
| Rou-w/o BC | Fixed C source ratio (A3C1), 180 rpm, round flask, no biochar | ||
| Rou-1% BC | Fixed C source ratio (A3C1), 180 rpm, round flask, 1% biochar | ||
| Reactor | C0-150 (Control) | Avicel:Cellulose = 4:0 (w/v, control), 150 rpm, no turbulence | |
| A3-180 | Fixed C source ratio (A3C1), 180 rpm, no turbulence | ||
| A3-180_Imp | Fixed C source ratio (A3C1), 180 rpm, impeller adjusted (turbulence added) | ||
| A3-210_Imp | Fixed C source ratio (A3C1), 210 rpm, impeller adjusted (turbulence added) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Myeong, S.; Lee, Y.-Y.; Yun, J. Optimization and Bioreactor Scale-Up of Cellulase Production in Trichoderma sp. KMF006 for Higher Yield and Performance. Int. J. Mol. Sci. 2025, 26, 3731. https://doi.org/10.3390/ijms26083731
Myeong S, Lee Y-Y, Yun J. Optimization and Bioreactor Scale-Up of Cellulase Production in Trichoderma sp. KMF006 for Higher Yield and Performance. International Journal of Molecular Sciences. 2025; 26(8):3731. https://doi.org/10.3390/ijms26083731
Chicago/Turabian StyleMyeong, Seongwoo, Yun-Yeong Lee, and Jeonghee Yun. 2025. "Optimization and Bioreactor Scale-Up of Cellulase Production in Trichoderma sp. KMF006 for Higher Yield and Performance" International Journal of Molecular Sciences 26, no. 8: 3731. https://doi.org/10.3390/ijms26083731
APA StyleMyeong, S., Lee, Y.-Y., & Yun, J. (2025). Optimization and Bioreactor Scale-Up of Cellulase Production in Trichoderma sp. KMF006 for Higher Yield and Performance. International Journal of Molecular Sciences, 26(8), 3731. https://doi.org/10.3390/ijms26083731






