Comparative Analysis of Physiological and Biochemical Responses to Salt Stress Reveals Important Mechanisms of Salt Tolerance in Wheat
Abstract
1. Introduction
2. Results
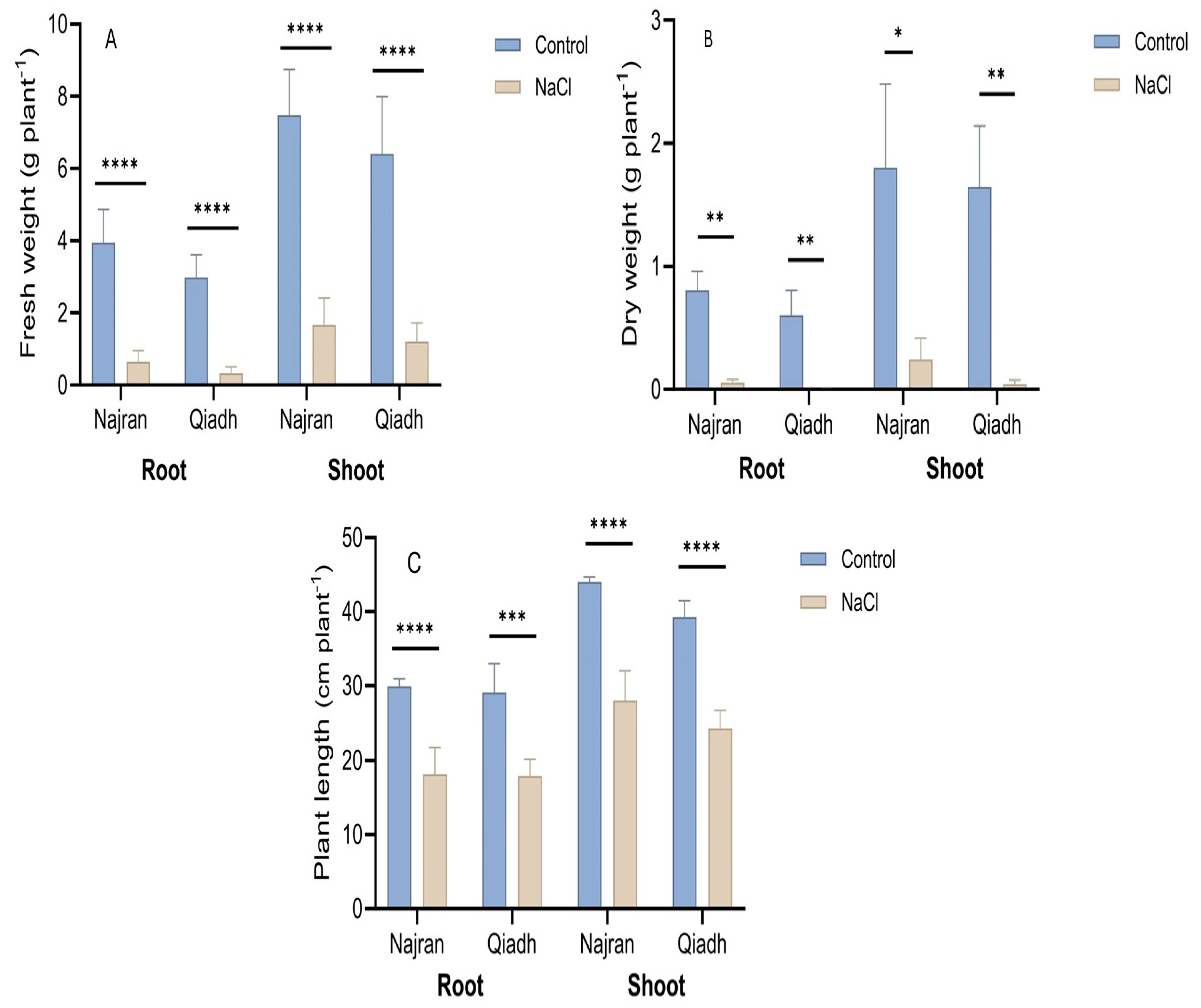
2.1. Plant Growth and Development
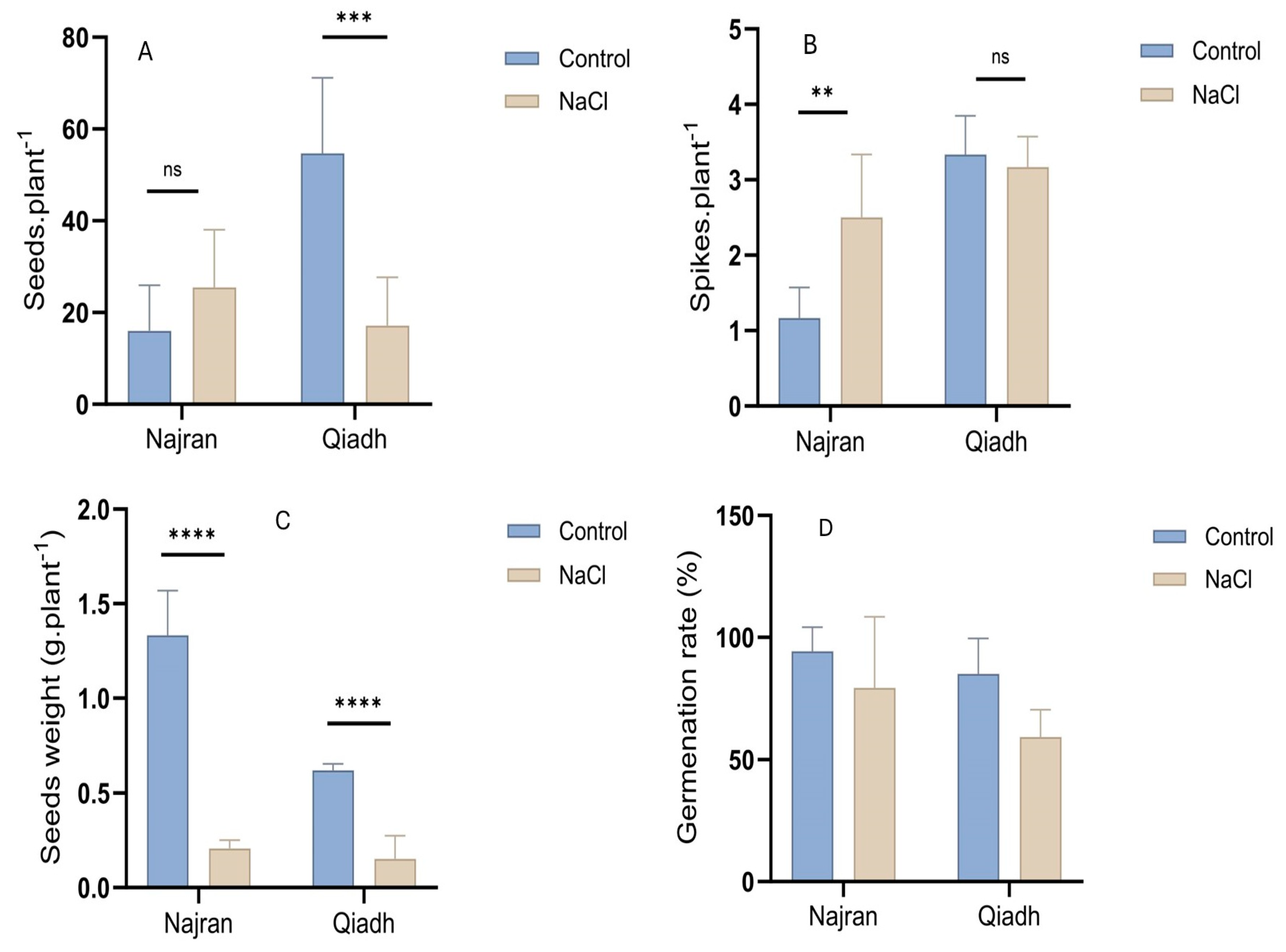
2.2. Grain Yield
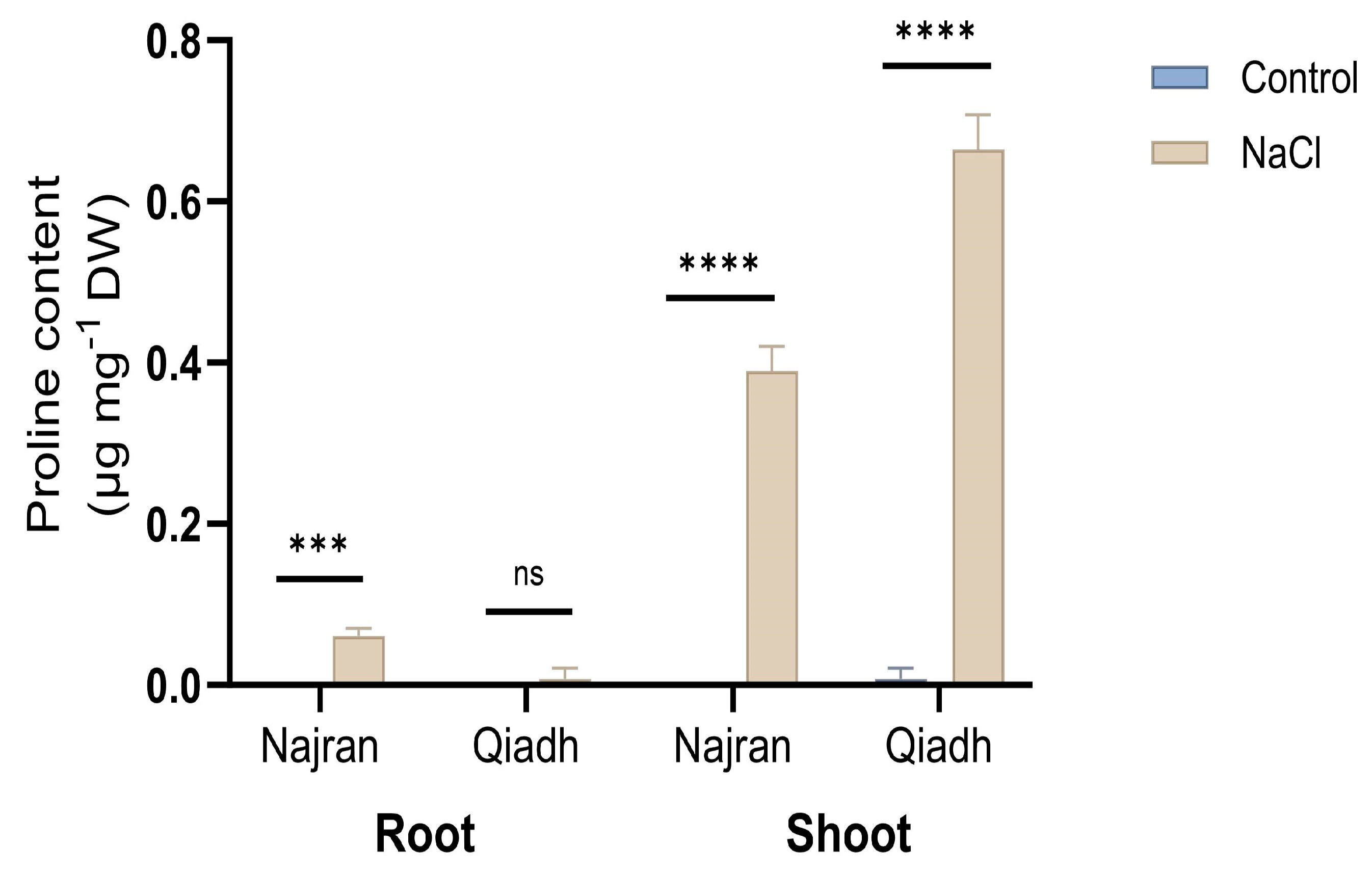
2.3. Proline Content
2.4. Soluble Sugars and Starch Levels
2.5. Total Organic Acids
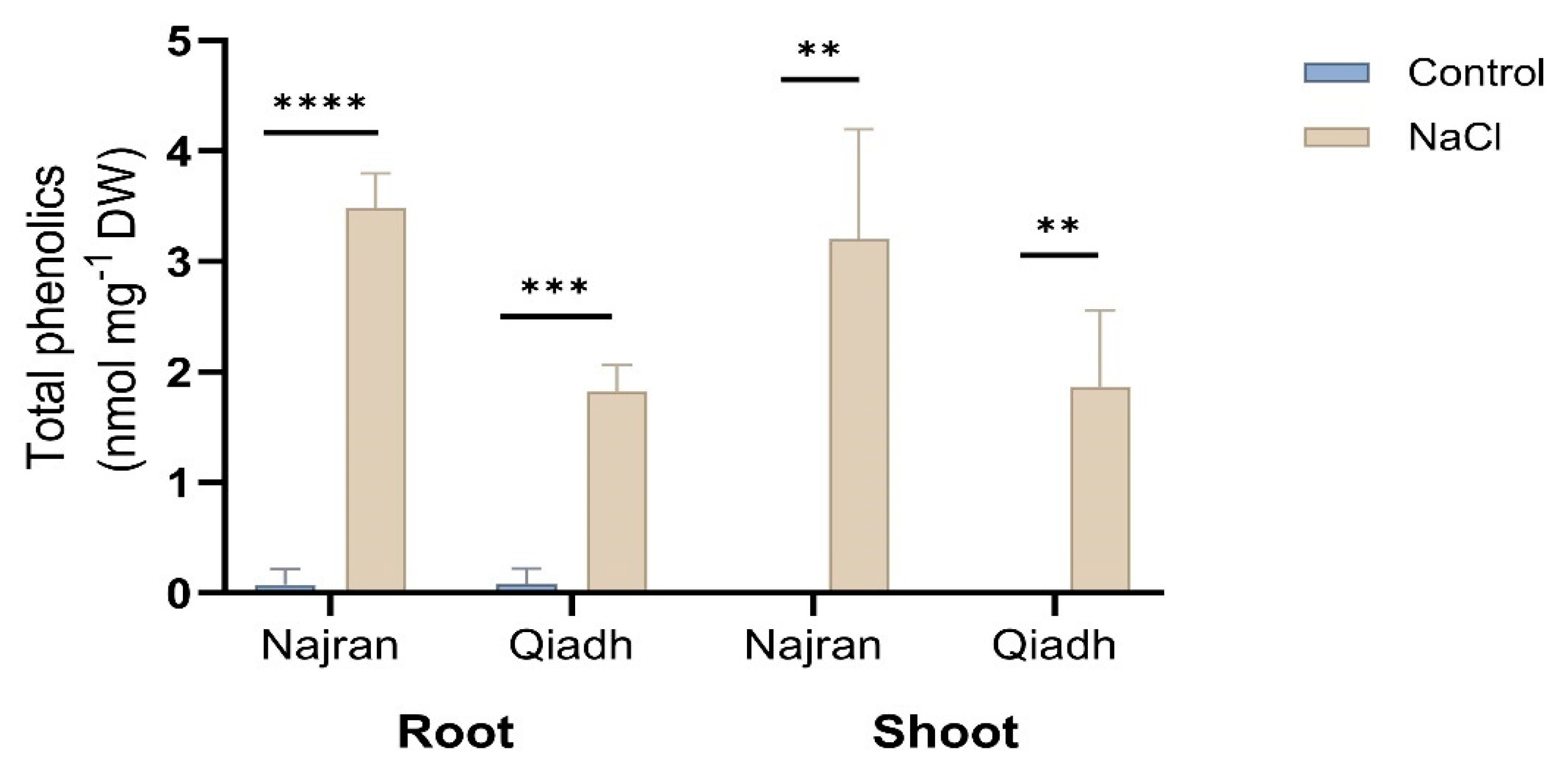
2.6. Phenolics Content
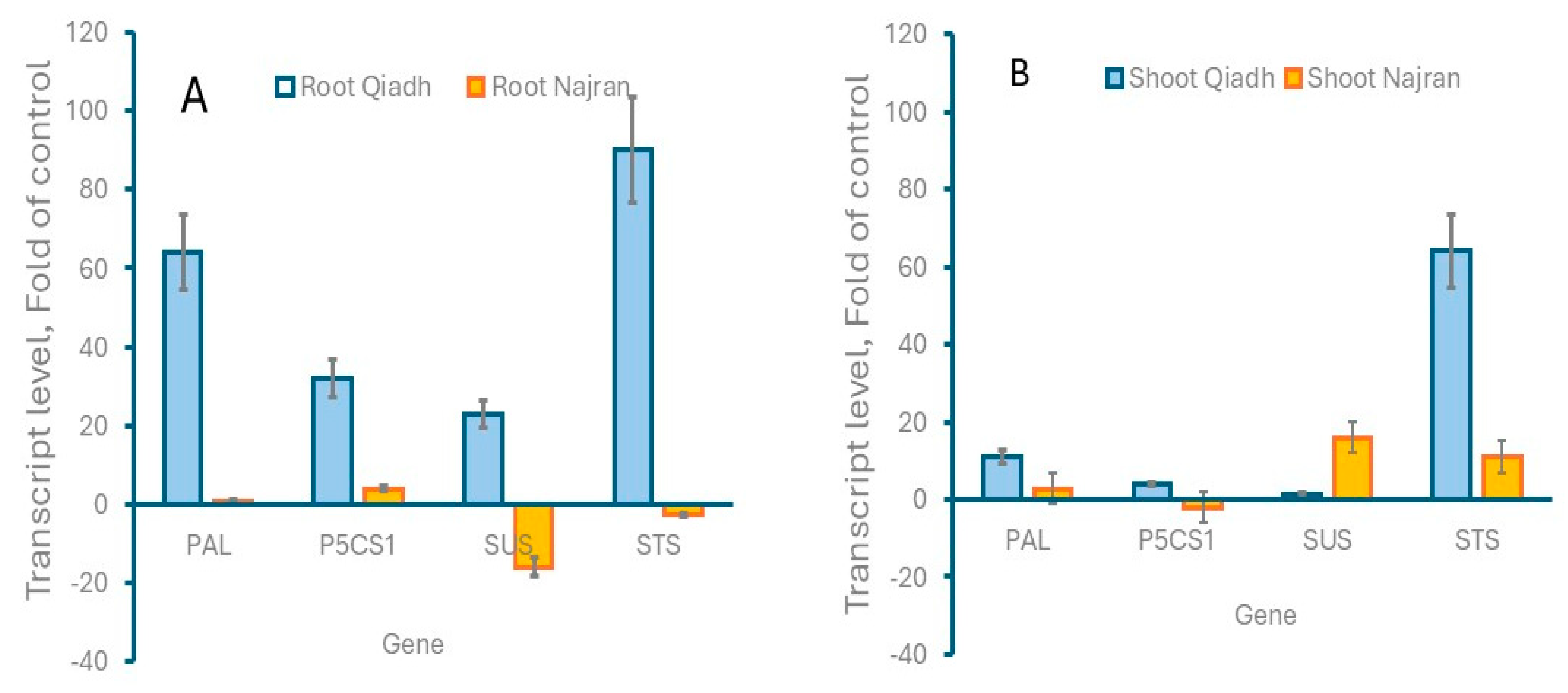
2.7. Gene Expression
3. Discussion
3.1. Plant Growth, Salt Stress Reduced Differentially the Growth of the Two Wheat Cultivars
3.2. Yield, Salt Stress Impacted Differentially the Spike and Seed Number and Seed Weight and Germination
3.3. Osmoregulation, Concomitant Production of Different Substances for Osmotic Adjustment Is Not Essentially Required for Salt Tolerance
3.4. Antioxidant, Salt Stress Enhanced Phenolics Production as an Antioxidant Response
4. Materials and Methods
4.1. Plant Materials and Salt Stress Treatment
4.2. Growth and Yield Analysis
4.3. Measurement of Proline Content
4.4. Measurement of Soluble Sugars and Starch Level
4.5. Measurement of Total Organic Acids
4.6. Measurement of Phenolics Content
4.7. RNA Extraction and qRT-PCR
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahsan, F.; Chandio, A.A.; Fang, W. Climate change impacts on cereal crops production in Pakistan: Evidence from cointegration analysis. Int. J. Clim. Change Strateg. Manag. 2020, 12, 257–269. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Wirth, S.; Bellingrath-Kimura, S.D.; Mishra, J.; Arora, N.K. Salt-Tolerant Plant Growth Promoting Rhizobacteria for Enhancing Crop Productivity of Saline Soils. Front. Microbiol. 2019, 10, 2791. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ha-Tran, D.M.; Nguyen, T.T.M.; Hung, S.H.; Huang, E.; Huang, C.C. Roles of Plant Growth-Promoting Rhizobacteria (PGPR) in Stimulating Salinity Stress Defense in Plants: A Review. Int. J. Mol. Sci. 2021, 22, 3154. [Google Scholar] [CrossRef]
- Saddiq, M.S.; Iqbal, S.; Hafeez, M.B.; Ibrahim, A.M.H.; Raza, A.; Fatima, E.M.; Baloch, H.; Jahanzaib, S.; Woodrow, P.; Ciarmiello, L.F. Effect of Salinity Stress on Physiological Changes in Winter and Spring Wheat. Agronomy 2021, 11, 1193. [Google Scholar] [CrossRef]
- Howladar, S.M.; Dennett, M. Improvement of Salt Tolerance in Saudi Arabian Wheat by Seed Priming or Foliar Spray with Salicylic Acid. Int. J. Agri. Biosyst. Eng. 2014, 8, 101–108. [Google Scholar]
- Munns, R.; Day, D.A.; FRricke, W.; Watt, M.; Arsova, B.; Barkla, B.J.; Bose, J.; Byrt, C.S.; Chen, Z.H.; Foster, K.J. Energy costs of salt tolerance in crop plants. New Phytol. 2020, 225, 1072–1090. [Google Scholar] [CrossRef]
- Wang, L.; Doan, P.P.T.; Chuong, N.N.; Lee, H.Y.; Kim, J.H.; Kim, J. Comprehensive transcriptomic analysis of age-, dark-, and salt-induced senescence reveals underlying mechanisms and key regulators of leaf senescence in Zoysia japonica. Front. Plant Sci. 2023, 14, 1170808. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sade, N.; Del Mar Rubio-Wilhelmi, M.; Umnajkitikorn, K.; Blumwald, E. Stress-induced senescence and plant tolerance to abiotic stress. J. Exp. Bot. 2018, 69, 845–853. [Google Scholar] [CrossRef] [PubMed]
- Ghoulam, C.; Foursy, A.; Fares, K. Effects of salt stress on growth, inorganic ions and proline accumulation in relation to osmotic adjustment in five sugar beet cultivars. Environ. Exp. Bot. 2002, 47, 39–50. [Google Scholar] [CrossRef]
- Meloni, D.A.; Olivia, M.A.; Ruiz, H.A.; Martinez, C.A. Contribution of proline and inorganic solutes to osmotic adjustment in cotton under salt stress. J. Plant Nutr. 2001, 24, 599–612. [Google Scholar] [CrossRef]
- Borrelli, G.M.; Fragasso, M.; Nigro, F.; Platani, C.; Papa, R.; Beleggia, R.; Trono, D. Analysis of metabolic and mineral changes in response to salt stress in durum wheat (Triticum turgidum ssp. durum) genotypes, which differ in salinity tolerance. Plant Physiol. Biochem. 2018, 133, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Farhangi-Abriz, S.; Torabian, S. Antioxidant enzyme and osmotic adjustment changes in bean seedlings as affected by biochar under salt stress. Ecotoxicol. Environ. Saf. 2017, 137, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Sun, X.; Wang, H.; Ma, X. Advances in salt tolerance molecular mechanism in tobacco plants. Hereditas 2020, 157, 5. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ayvaz, M.; Guven, A.; Blokhina, O.; Fagersdedt, K.V. Boron stress, oxidative damage and antioxidant protection in potato cultivars (Solanum tuberosum L.). Acta Agricult. Scand. Sect. B—Soil Plant Sci. 2016, 66, 302–316. [Google Scholar]
- Li, L.; Peng, Z.; Mao, X.; Wang, J.; Li, C.; Chang, X.; Jing, R. Genetic insights into natural variation underlying salt tolerance in wheat. J. Exp. Bot. 2021, 72, 1135–1150. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Qiao, W.; Liu, X.; Shi, J.; Xu, Q.; Zhou, H.; Yan, G.; Huang, Q. Relative contribution of Na+/K+ homeostasis, photochemical efficiency and antioxidant defense system to differential salt tolerance in cotton (Gossypium hirsutum L.) cultivars. Plant Physiol. Biochem. 2017, 119, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Wingen, L.U.; West, C.; Leverington-Waite, M.; Collier, S.; Orford, S.; Goram, R.; Yang, C.Y.; King, J.; Allen, A.M.; Burridge, A.; et al. Wheat Landrace Genome Diversity. Genetics 2017, 205, 1657–1676. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ghonaim, M.M.; Mohamed, H.I.; Omran, A.A. Evaluation of wheat (Triticum aestivum L.) salt stress tolerance using physiological parameters and retrotransposon-based markers. Genet. Resour. Crop Evol. 2021, 68, 227–242. [Google Scholar] [CrossRef]
- Tao, R.; Ding, J.; Li, C.; Zhu, X.; Guo, W.; Zhu, M. Evaluating and Screening of Agro-Physiological Indices for Salinity Stress Tolerance in Wheat at the Seedling Stage. Front. Plant Sci. 2021, 12, 646175. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zeesham, M.; Lu, M.; Sehar, S.; Holford, P.; Wu, F. Comparison of biochemical, anatomical, morphological, and physiological responses to salinity stress in wheat and barley genotypes deferring in salinity tolerance. Agronomy 2020, 10, 127. [Google Scholar] [CrossRef]
- Boutraa, T.; Akhkha, A.; AL-shoaibi, A.A.; Alhejeli, A.M. Effect of water stress on growth and water use efficiency (WUE) of some wheat cultivars (Triticum durum) grown in Saudi Arabia. J. Taibah Univ. Sci. 2010, 3, 39–48. [Google Scholar] [CrossRef]
- Akhkha, A.; Boutraa, T.; Alhejeli, A. The rates of photosynthesis, chlorophyll content, dark respiration, proline and abscicic acid (ABA) in wheat (Triticum durum) under water deficit conditions. Int. J. Agri. Biol. 2011, 13, 1560. [Google Scholar]
- Albokari, M.M.; Khashoggi, A.J.; Almuwalid, M.A. Effect of different irrigated conditions on some morphological traits of wheat genotypes grown in Saudi Arabia. Pak. J. Bot. 2016, 48, 519–526. [Google Scholar]
- Boutraa, T.; Akhkha, A.; AL-Shoaibi, A.K. Evaluation of growth and gas exchange rates of two local saudi wheat cultivars grown under heat stress conditions. Pak. J. Bot. 2015, 47, 27–34. [Google Scholar]
- Dawabah, A.; AL-Hamzi, A.S.; AL-Yahya, F.A. Management of cereal cyst nematode (Heterodera avenae) in a large scale wheat production. Nematodes Small Grain Cereals 2015, 277–284. [Google Scholar]
- Albokari, M.M.; Almuwalid, M.A. Evaluation of some local wheat landraces treated with different doses of gamma rays in Saudi Arabia. Pak. J. Biotech. 2015, 12, 63–72. [Google Scholar]
- Howladar, S.M. Effects of Salicylic Acid on Salinity Tolerance of Wheat (Triticum aestivum). Ph.D. Thesis, University of Reading, Reading, UK, 2010. [Google Scholar]
- Almaghrabi, O.A. Response of Saudi and Egyptian wheat cultivars to salinity stress during germination. J. Food Agric. Environ. 2012, 10, 1334–1338. [Google Scholar]
- Alshaharni, M.O. Molecular, Biochemical and Physiological Responses of Wheat (Triticum aestivum) to Spot Blotch Disease and Salinity. Ph.D. Thesis, Newcastle University, Newcastle upon Tyne, UK, 2022. [Google Scholar]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant. Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed]
- Gupta, B.; Huang, B. Mechanism of salinity tolerance in plants: Physiological, biochemical, and molecular characterization. Int. J. Genom. 2014, 2014, 701596. [Google Scholar] [CrossRef]
- Asheaf, M.A.; Ashraf, M.; Ali, Q. Response of two genetically diverse wheat cultivars to salt stress at different growth stages: Leaf lipid peroxidation and phenolic contents. Pak. J. Bot. 2010, 42, 559–565. [Google Scholar]
- Chavarria, G.; Dos Santos, H.P. Plant water relations: Absorption, transport and control mechanisms. In Advances in Selected Plant Physiology Aspects; InTech: Tokyo, Japan, 2012; pp. 105–132. [Google Scholar] [CrossRef]
- Munns, R. Comparative physiology of salt and water stress. Plant Cell Environ. 2002, 25, 239–250. [Google Scholar] [CrossRef]
- Taleisnik, E.; Rodríguez, A.A.; Bustos, D.; Erdei, L.; Ortega, L.; Senn, M.E. Leaf expansion in grasses under salt stress. J. Plant Physiol. 2009, 166, 1123–1140. [Google Scholar] [CrossRef]
- Nadolska-Orckzyk, A.; Rajchel, I.K.; Orczy, W.; Gasparis, S. Major genes determining yield-related traits in wheat and barley. Theor. Appl. Genet. 2017, 130, 1081–1098. [Google Scholar] [CrossRef]
- Hasegawa, P.M.; Bressan, R.A.; Zhu, J.K.; Bohnert, H.J. Plant cellular and molecular responses to high salinity. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2000, 51, 463–499. [Google Scholar] [CrossRef] [PubMed]
- Verbruggen, N.; Hermans, C. Proline accumulation in plants: A review. Amino Acids 2008, 35, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Hare, P.; Cress, W. Metabolic implications of stress-induced proline accumulation in plants. Plant Growth Regul. 1997, 21, 79–102. [Google Scholar] [CrossRef]
- Szabados, L.; Savouré, A. Proline: A multifunctional amino acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.S.; Jogeswar, G.; Rasineni, G.K.; Maheswari, M.; Reddy, A.R.; Varshney, R.K.; Kavi Kishor, P.B. Proline over-accumulation alleviates salt stress and protects photosynthetic and antioxidant enzyme activities in transgenic sorghum [Sorghum bicolor (L.) Moench]. Plant Physiol. Biochem. 2015, 94, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Maggio, A.; Miyazaki, S.; Veronese, P.; Fujita, T.; Ibeas, J.I.; Damsz, B.; Narasimhan, M.L.; Hasegawa, P.M.; Joly, R.J.; Bressan, R.A. Does proline accumulation play an active role in stress-induced growth reduction? Plant J. 2002, 31, 699–712. [Google Scholar] [CrossRef] [PubMed]
- Boriboonkaset, T.; Theerawitaya, C.; Yamada, N.; Pichakum, A.; Supaibulwatana, K.; Cha-Um, S.; Takabe, T.; Kirdmanee, C. Regulation of some carbohydrate metabolism-related genes, starch and soluble sugar contents, photosynthetic activities and yield attributes of two contrasting rice genotypes subjected to salt stress. Protoplasma 2013, 250, 1157–1167. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Chen, H.; Fan, J.; Wang, Y.; Li, Y.; Chen, J.; Fan, J.; Yang, S.; Hu, L.; Leung, H.; et al. Genetic diversity and disease control in rice. Nature 2000, 17, 718–722. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wang, W.; Zhang, F.; Deng, J.; Li, Z.; Fu, B. Comparative metabolite profiling of two rice genotypes with contrasting salt stress tolerance at the seedling stage. PLoS ONE 2014, 9, e108020. [Google Scholar] [CrossRef]
- Hernandez, J.A.; Jimenez, A.; Mullineaux, P.; Sevilla, F. Tolerance of pea (Pisum sativum L.) to long-term salt stress is associated with induction of antioxidant defences. Plant Cell Environ. 2000, 23, 853–862. [Google Scholar] [CrossRef]
- Sairam, R.; Srivastava, G.; Saxena, D. Increased antioxidant activity under elevated temperatures: A mechanism of heat stress tolerance in wheat genotypes. Biol. Plant 2000, 43, 245–251. [Google Scholar] [CrossRef]
- Zhou, R.; Kong, L.; Yu, X.; Ottosen, C.O.; Zhao, T.; Jiang, F.; Wu, Z. Oxidative damage and antioxidant mechanism in tomatoes responding to drought and heat stress. Acta Physiol. Plant. 2019, 41, 20. [Google Scholar] [CrossRef]
- Alyahya, N.; Taybi, T. Comparative transcriptomic profiling reveals differentially expressed genes and important related metabolic pathways in shoots and roots of a Saudi wheat cultivar (Najran) under salinity stress. Front. Plant Sci. 2023, 14, 1225541. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Claussen, W. Proline as a measure of stress in tomato plants. Plant Sci. 2005, 168, 241–248. [Google Scholar] [CrossRef]
- Taybi, T.; Cushman, J. Signaling Events Leading to Crassulacean Acid Metabolism Induction in the Common Ice Plant. Plant Physiol. 1999, 121, 545–556. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]


| Gene | Primers | Melting Temperature (°C) | Efficiency (%) | Amplicon Size (bp) | GenBank, Number |
|---|---|---|---|---|---|
| PAL | Forward: 5′TAGTGTTGGGTAGCCAGTAGA3′ | 62 | 97.5 | 136 | X99725.1 |
| Reverse: 5′AGTTGGTTCTCGGACTATTGC3′ | 62 | ||||
| P5CS1 | Forward: 5′CGCGAAACTGTCGAGTCATTAT3′ | 63 | 97.2 | 130 | XM_048685323 |
| Reverse: 5′ACCTGCCAAACTGTCATTATCC3′ | 63 | ||||
| SUS | Forward:5′GAAGTACGTGAGCAACCTAGAG3′ | 62 | 98 | 105 | XM_048673430.1 |
| Reverse: 5′TCAACCGCCAATGGAACT 3′ | 62 | ||||
| STS | Forward: 5′ACGTGCTTCTGGAACTGG3′ | 62 | 97.4 | 120 | AY050174.1 |
| Reverse: 5′CCAGAAGCTCCTCTTCTTTCTC3′ | 63 | ||||
| GAPDH | Forward: 5′AACGACCCCTTCATCACCAC3′ | 65 | 98 | 150 | EF592180 |
| Reverse: 5′GTTCCTGCAGCCAAACACAG3′ | 65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taybi, T.; Alyahya, N. Comparative Analysis of Physiological and Biochemical Responses to Salt Stress Reveals Important Mechanisms of Salt Tolerance in Wheat. Int. J. Mol. Sci. 2025, 26, 3742. https://doi.org/10.3390/ijms26083742
Taybi T, Alyahya N. Comparative Analysis of Physiological and Biochemical Responses to Salt Stress Reveals Important Mechanisms of Salt Tolerance in Wheat. International Journal of Molecular Sciences. 2025; 26(8):3742. https://doi.org/10.3390/ijms26083742
Chicago/Turabian StyleTaybi, Tahar, and Norah Alyahya. 2025. "Comparative Analysis of Physiological and Biochemical Responses to Salt Stress Reveals Important Mechanisms of Salt Tolerance in Wheat" International Journal of Molecular Sciences 26, no. 8: 3742. https://doi.org/10.3390/ijms26083742
APA StyleTaybi, T., & Alyahya, N. (2025). Comparative Analysis of Physiological and Biochemical Responses to Salt Stress Reveals Important Mechanisms of Salt Tolerance in Wheat. International Journal of Molecular Sciences, 26(8), 3742. https://doi.org/10.3390/ijms26083742





