Gut Microbiota Metabolites and Chronic Diseases: Interactions, Mechanisms, and Therapeutic Strategies
Abstract
1. Introduction
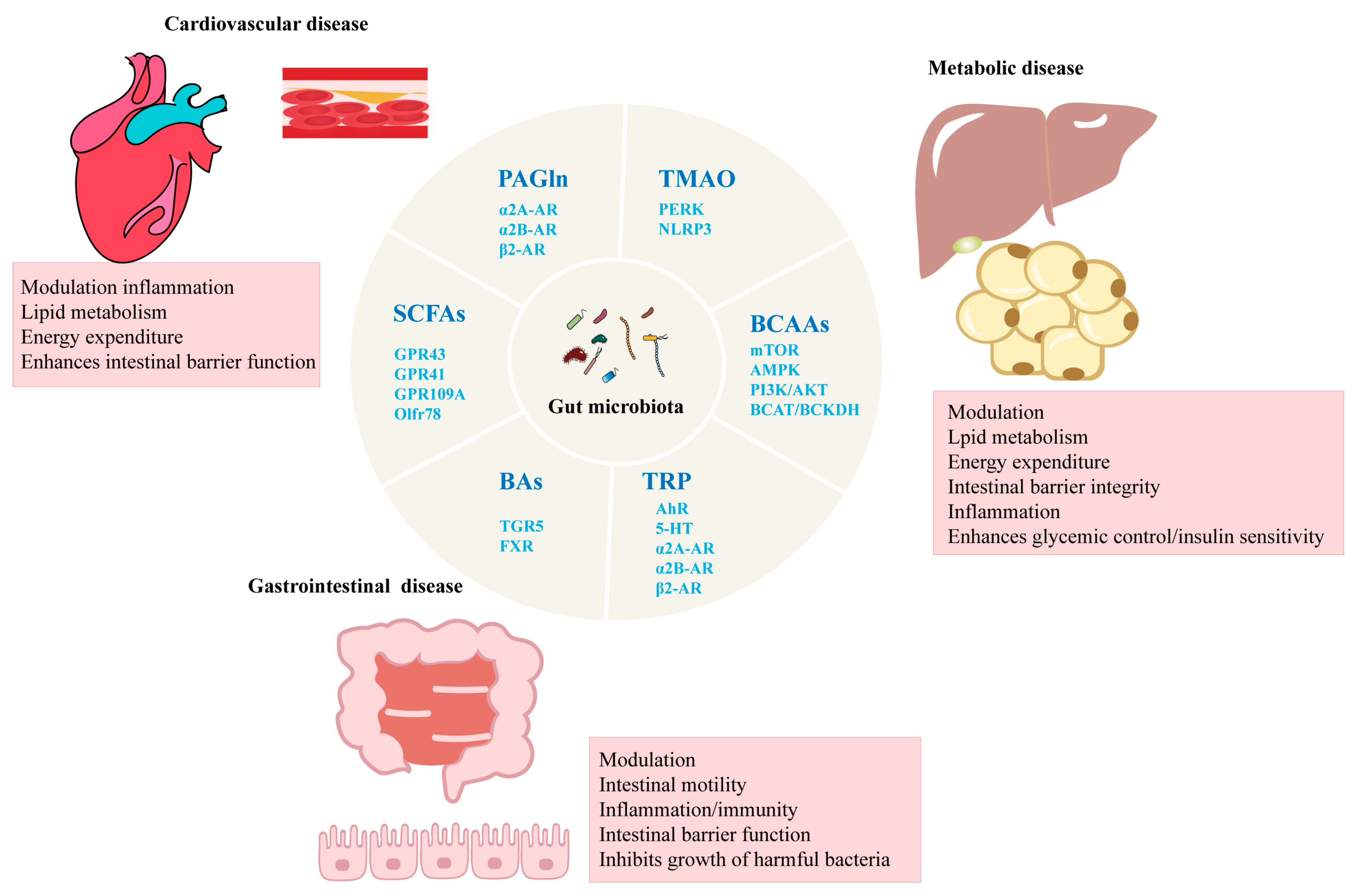
2. Gut Microbiota and Metabolites
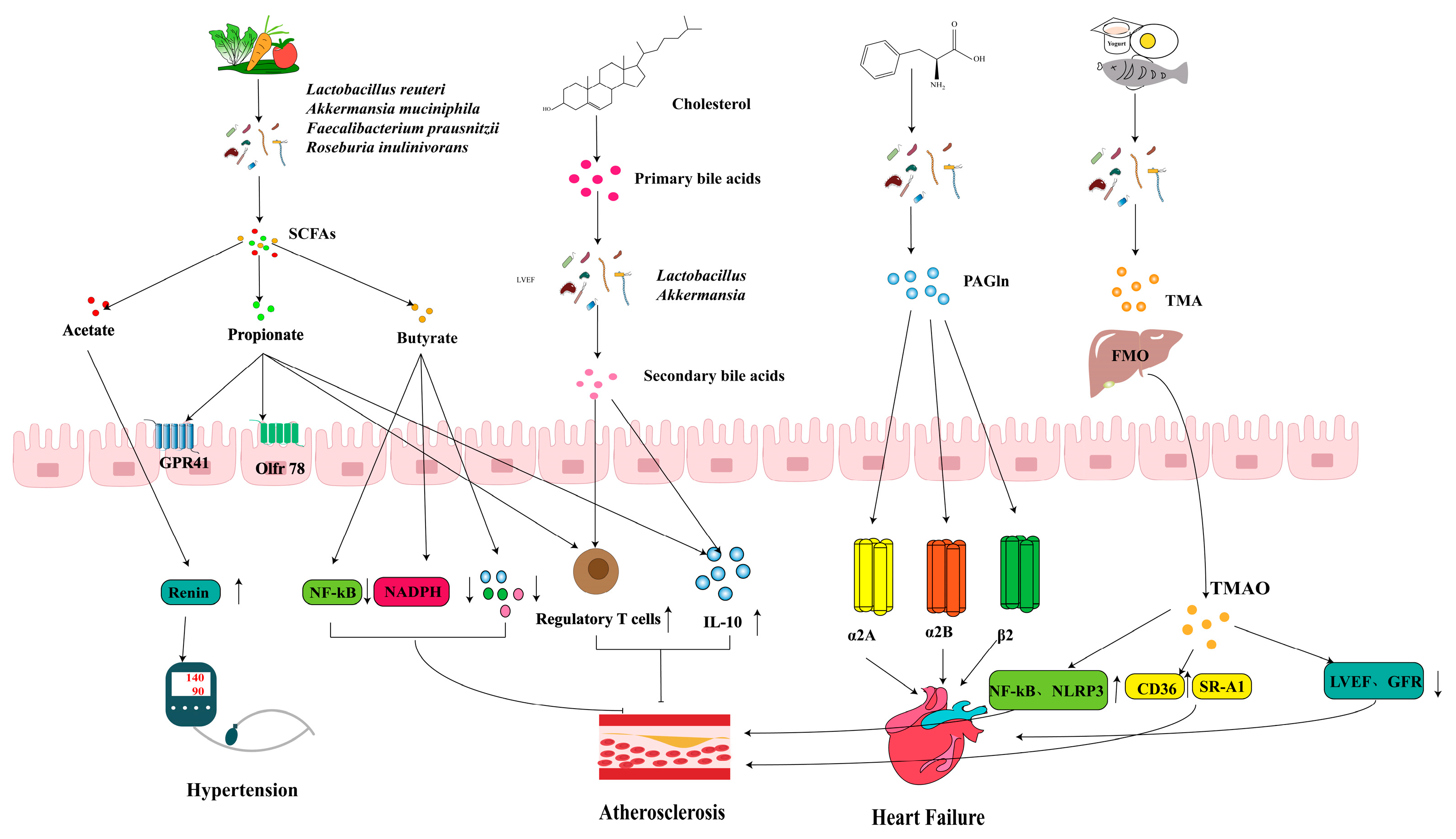
2.1. TMAO
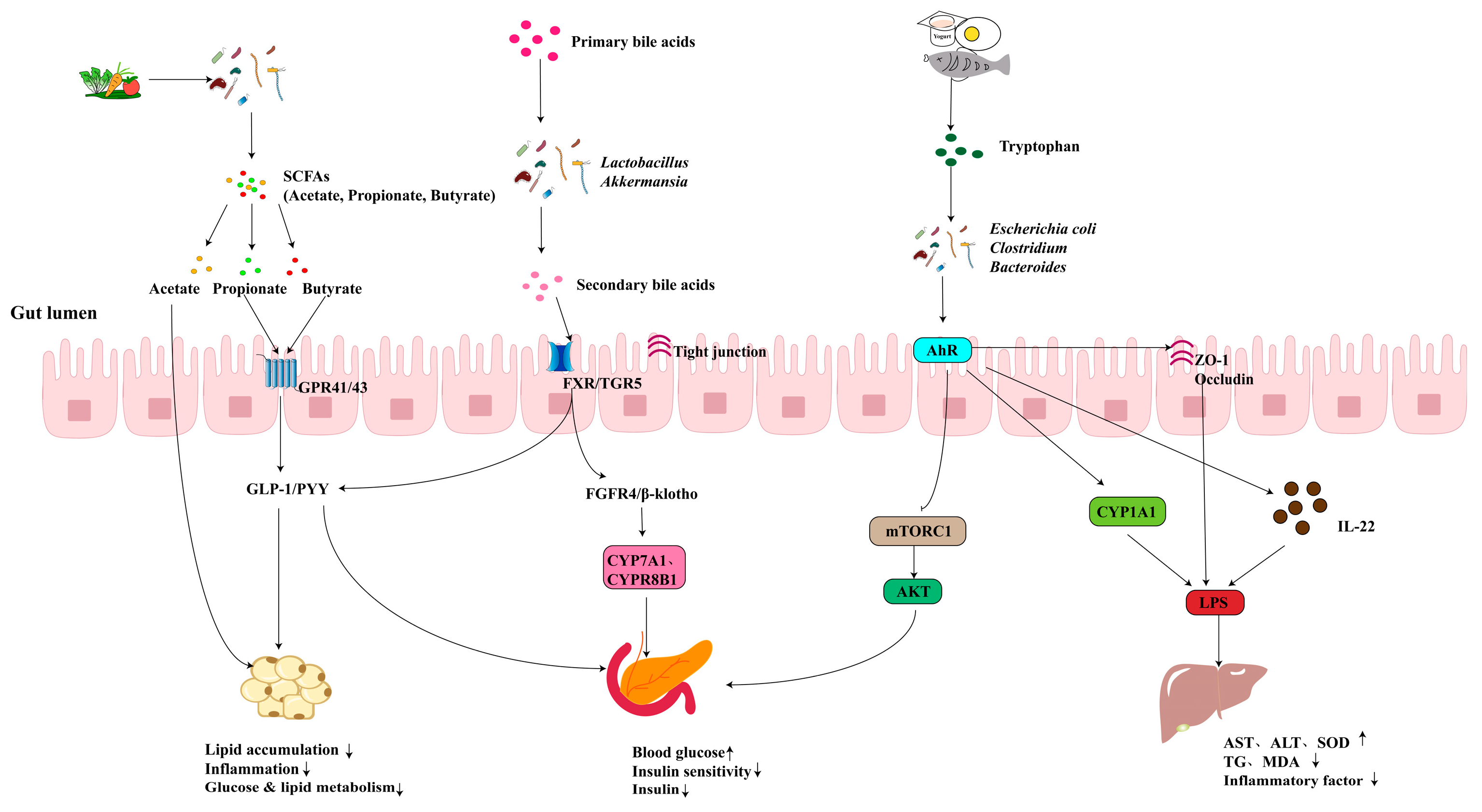
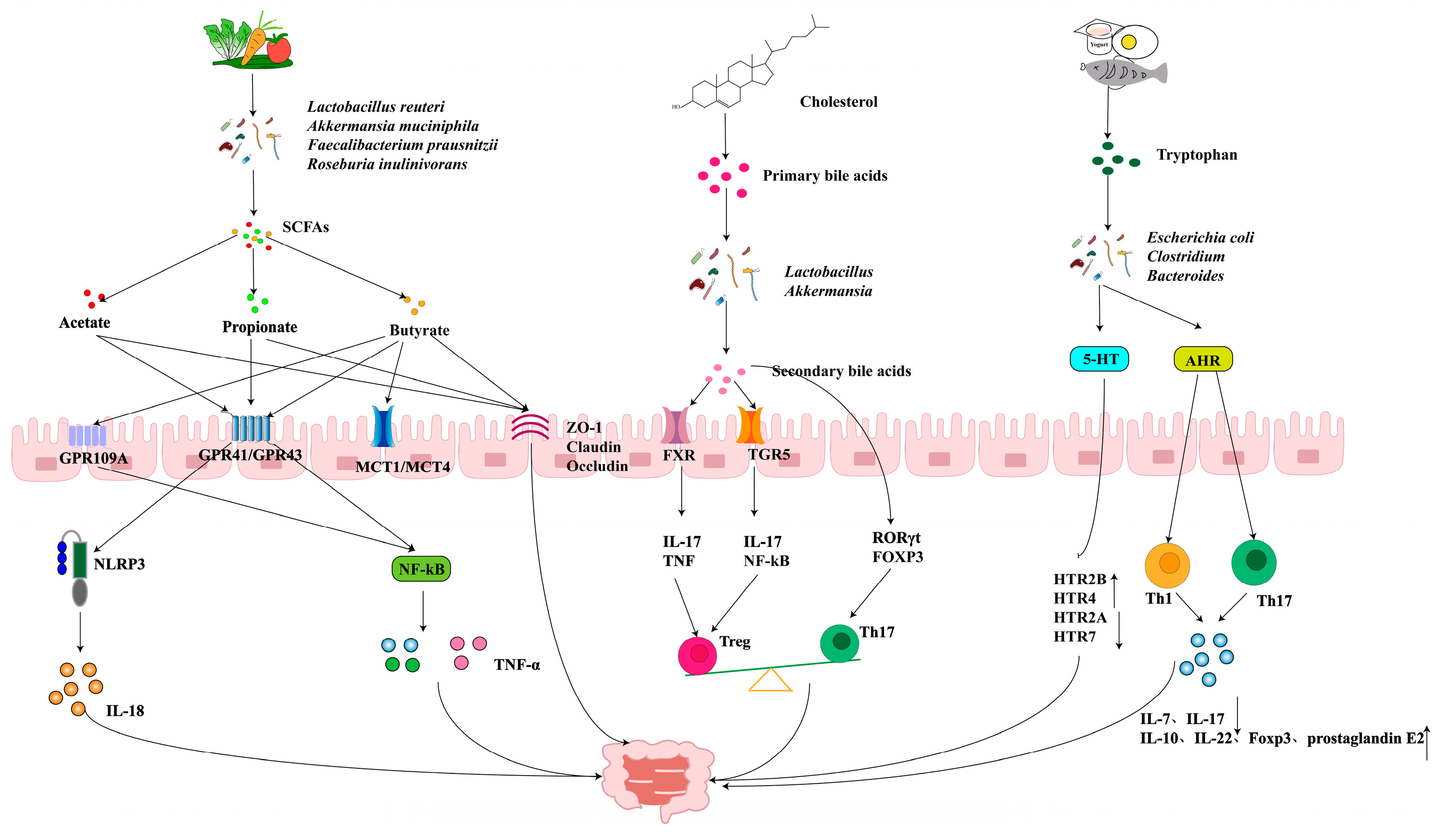
2.2. SCFAs
2.3. BAs
2.4. PAGln
2.5. BCAAs
2.6. TRP
3. The Relationship Between Gut Microbiota and Chronic Diseases
3.1. CVD
3.1.1. HFN
3.1.2. AS
3.1.3. HF
3.2. Metabolic Disease
3.2.1. Obesity
3.2.2. T2D
3.2.3. MASLD
3.3. Gastrointestinal Diseases
3.3.1. BAs and IBD
3.3.2. SCFAs in IBD
3.3.3. AHR and Its Role in IBD
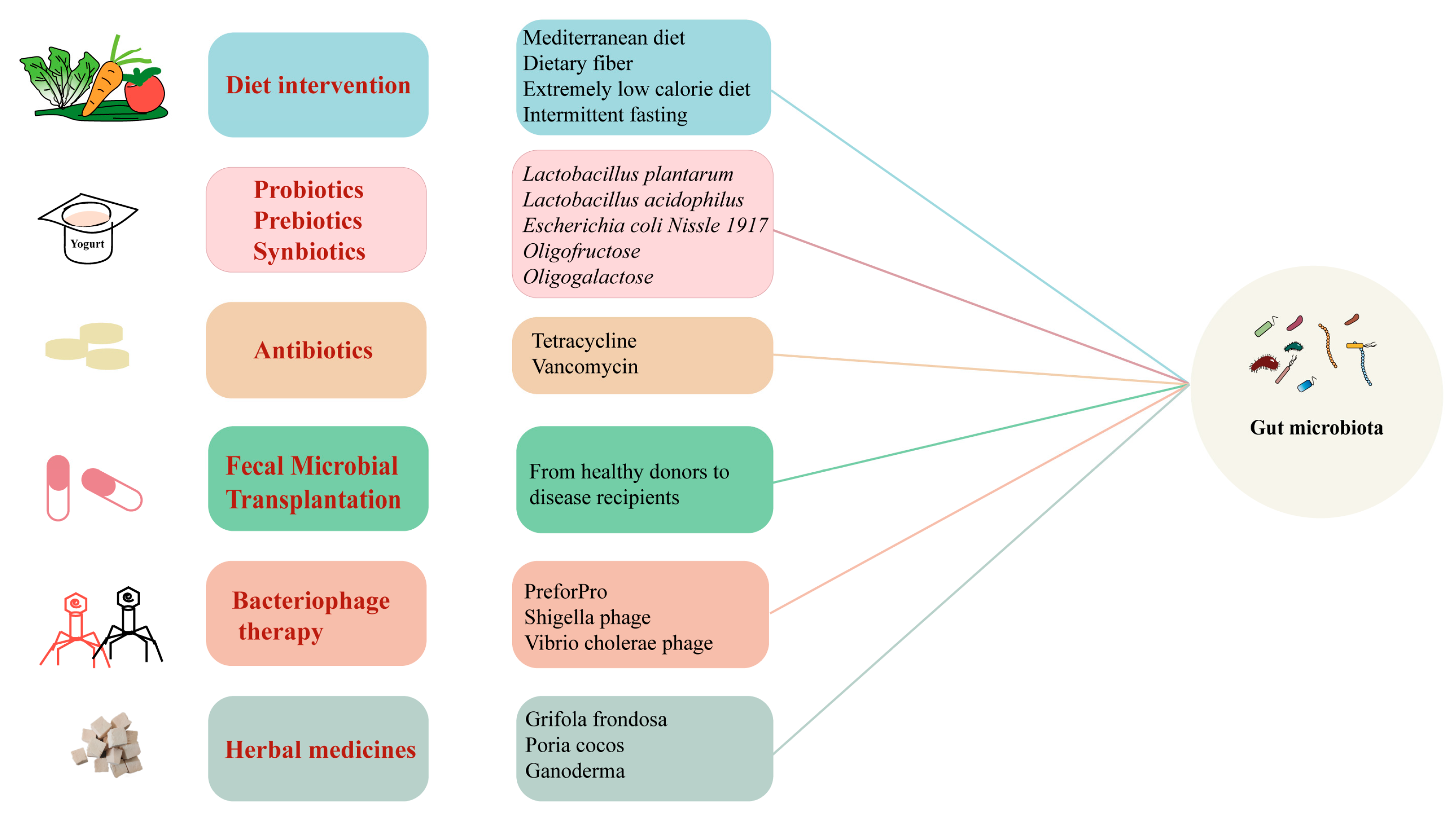
4. Targeted “Gut Microbiota” Therapies
4.1. Dietary Intervention
4.2. Probiotic, Prebiotic, and Synbiotic Interventions
4.3. Antibiotics
4.4. FMT and Phage Therapy
5. Future Outlook
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CVD | cardiovascular disease |
| HTN | hypertension |
| AS | atherosclerosis |
| HF | heart failure |
| T2D | type 2 diabetes |
| MASLD | metabolic dysfunction-associated steatotic liver disease |
| IBD | inflammatory bowel disease |
| CRC | colorectal cancer |
| NCDs | non-communicable diseases |
| TMAO | trimethylamine N-oxide |
| TMA | trimethylamine |
| SCFAs | short-chain fatty acids |
| BAs | bile acids |
| PAGln | phenylacetylglutamine |
| BCAAs | branched-chain amino acids |
| TRP | tryptophan |
| FMT | fecal microbiota transplantation |
| FMOs | flavin-containing monooxygenases |
| GPRs | G protein-coupled receptors |
| CDCA | chenodeoxycholic acid |
| FXR | Farnesoid X Receptor |
| TGR5 | G protein-coupled bile acid receptor |
| 5-HT | serotonin |
| PVN | paraventricular nucleus |
| ANG II | angiotensin II |
| RAS | renin-angiotensin system |
| ACE2 | Angiotensin-converting enzyme 2 |
| HMGB1 | high-mobility group box 1 |
| TLR | Toll-like receptor |
| NF-κB | nuclear factor kappa-B |
| mTORC1 | mechanistic target of rapamycin complex 1 |
| CD36 | cluster of differentiation 36 |
| SR-A1 | scavenger receptor A1 |
| MAPK | mitogen-activated protein kinase |
| NLRP3 | nucleotide-binding oligomerization domain-like receptor protein 3 |
| LVEF | left ventricular ejection fraction |
| GFR | glomerular filtration rate |
| AHR | aryl hydrocarbon receptor |
| IL-22 | interleukin-22 |
| GLP-1 | glucagon-like peptide-1 |
| KD | ketogenic diet |
| TDCA | taurodeoxycholic acid |
| TUDCA | tauroursodeoxycholic acid |
| NAFLD | non-alcoholic fatty liver disease |
| NASH | non-alcoholic steatohepatitis |
| FASN | fatty acid synthase |
| IPA | indole-3-propionic acid |
| IAA | indole-2-acetic acid |
| CD | Crohn’s disease |
| UC | ulcerative colitis |
| CDCA | chenodeoxycholic acid |
| Th17 | T-helper 17 |
| Treg | regulatory T |
| TNF | tumor necrosis factor |
| HDAC | histone deacetylase |
| IL-17 | interleukin 17 |
| Foxp3 | forkhead box p3 |
| ODC1 | ornithine decarboxylase |
| ILA | indole-3-lactic acid |
References
- Yang, Z.-Y.; Tang, J.-L. Definitions of Chronic Disease Need to Be More Patient Centred. BMJ 2024, q1858. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Pedersen, O. Gut Microbiota in Human Metabolic Health and Disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wang, K.; Wang, X.; Pang, Y.; Jiang, C. The Role of the Gut Microbiome and Its Metabolites in Metabolic Diseases. Protein Cell 2021, 12, 360–373. [Google Scholar] [CrossRef]
- Xie, Q. Effect of Coptis Chinensis Franch and Magnolia Officinalis on Intestinal Flora and Intestinal Barrier in a TNBS-Induced Ulcerative Colitis Rats Model. Phytomedicine 2022, 97, 153927. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, M.; Yang, M.; Jin, C.; Song, Y.; Chen, J.; Gao, M.; Ai, Z.; Su, D. Pulsatilla Chinensis Saponins Ameliorate Inflammation and DSS-Induced Ulcerative Colitis in Rats by Regulating the Composition and Diversity of Intestinal Flora. Front. Cell. Infect. Microbiol. 2021, 11, 728929. [Google Scholar] [CrossRef]
- Chen, Y. Role and Mechanism of Gut Microbiota in Human Disease. Front. Cell Infect. Microbiol. 2021, 11, 625913. [Google Scholar] [CrossRef]
- Agus, A.; Clément, K.; Sokol, H. Gut Microbiota-Derived Metabolites as Central Regulators in Metabolic Disorders. Gut 2021, 70, 1174–1182. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S. Gut Microbiota Dependent Trimethylamine N-Oxide Aggravates Angiotensin II–Induced Hypertension. Redox Biol. 2021, 46, 102115. [Google Scholar] [CrossRef]
- Zou, Y.; Song, X.; Liu, N.; Sun, W.; Liu, B. Intestinal Flora: A Potential New Regulator of Cardiovascular Disease. Aging Dis. 2022, 13, 753. [Google Scholar] [CrossRef]
- Li, X.S.; Obeid, S.; Klingenberg, R.; Gencer, B.; Mach, F.; Räber, L.; Windecker, S.; Rodondi, N.; Nanchen, D.; Muller, O.; et al. Gut Microbiota-Dependent Trimethylamine N-Oxide in Acute Coronary Syndromes: A Prognostic Marker for Incident Cardiovascular Events beyond Traditional Risk Factors. Eur. Heart J. 2017, 38, ehw582. [Google Scholar] [CrossRef]
- Bi, S.-H.; Su, C.; Yang, P.; Zhang, X.; Wang, Y.; Tang, W.; Yang, W.; He, L. Higher Serum Trimethylamine N-Oxide (TMAO) Levels Are Associated with Increased Visceral Fat in Hemodialysis Patients. CN 2023, 100, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Zhao, Q.; Jiang, X.; Hu, J.; Jiang, Q.; Sheng, L.; Peng, X.; Wang, S.; Chen, Y.; Wan, Y.; et al. Trimethylamine N-Oxide Impairs β-Cell Function and Glucose Tolerance. Nat. Commun. 2024, 15, 2526. [Google Scholar] [CrossRef]
- Nian, F.; Chen, Y.; Xia, Q.; Zhu, C.; Wu, L.; Lu, X. Gut Microbiota Metabolite Trimethylamine N-Oxide Promoted NAFLD Progression by Exacerbating Intestinal Barrier Disruption and Intrahepatic Cellular Imbalance. Int. Immunopharmacol. 2024, 142, 113173. [Google Scholar] [CrossRef] [PubMed]
- Yue, C.; Yang, X.; Li, J.; Chen, X.; Zhao, X.; Chen, Y.; Wen, Y. Trimethylamine N-Oxide Prime NLRP3 Inflammasome via Inhibiting ATG16L1-Induced Autophagy in Colonic Epithelial Cells. Biochem. Biophys. Res. Commun. 2017, 490, 541–551. [Google Scholar] [CrossRef]
- Hsu, C.-N.; Yu, H.-R.; Lin, I.-C.; Tiao, M.-M.; Huang, L.-T.; Hou, C.-Y.; Chang-Chien, G.-P.; Lin, S.; Tain, Y.-L. Sodium Butyrate Modulates Blood Pressure and Gut Microbiota in Maternal Tryptophan-Free Diet-Induced Hypertension Rat Offspring. J. Nutr. Biochem. 2022, 108, 109090. [Google Scholar] [CrossRef]
- Wang, Y.; Dou, W.; Qian, X.; Chen, H.; Zhang, Y.; Yang, L.; Wu, Y.; Xu, X. Advancements in the Study of Short-Chain Fatty Acids and Their Therapeutic Effects on Atherosclerosis. Life Sci. 2025, 369, 123528. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, N.; Kobayashi, M.; Ito, M.; Matsui, H.; Ohashi, K.; Murohara, T.; Takeda, J.; Ueyama, J.; Hirayama, M.; Ohno, K. Soy Protein β-Conglycinin Ameliorates Pressure Overload-Induced Heart Failure by Increasing Short-Chain Fatty Acid (SCFA)-Producing Gut Microbiota and Intestinal SCFAs. Clin. Nutr. 2024, 43, 124–137. [Google Scholar] [CrossRef]
- Su, C.-W.; Chen, C.-Y.; Mao, T.; Chen, N.; Steudel, N.; Jiao, L.; Lan, J.; Fasano, A.; Walker, W.A.; Shi, H.N. Maternal Helminth Infection Protects Offspring from High-Fat-Diet-Induced Obesity through Altered Microbiota and SCFAs. Cell Mol. Immunol. 2023, 20, 389–403. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, F.; Ding, X.; Wu, G.; Lam, Y.Y.; Wang, X.; Fu, H.; Xue, X.; Lu, C.; Ma, J.; et al. Gut Bacteria Selectively Promoted by Dietary Fibers Alleviate Type 2 Diabetes. Science 2018, 359, 1151–1156. [Google Scholar] [CrossRef]
- Hong, Y.; Sheng, L.; Zhong, J.; Tao, X.; Zhu, W.; Ma, J.; Yan, J.; Zhao, A.; Zheng, X.; Wu, G.; et al. Desulfovibrio Vulgaris, a Potent Acetic Acid-Producing Bacterium, Attenuates Nonalcoholic Fatty Liver Disease in Mice. Gut Microbes 2021, 13, 1930874. [Google Scholar] [CrossRef]
- Parada Venegas, D.; De La Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef]
- Li, Y.; Gao, Y.-N.; Zhu, Y.-B.; Lu, W.-F.; Yu, J.-Y.; Dong, Y.-Y.; Xu, M.-Y.; Peng, B.; Wu, J.-Z.; Su, Q.; et al. Taurocholic Acid Ameliorates Hypertension through the Activation of TGR5 in the Hypothalamic Paraventricular Nucleus. Food Funct. 2024, 15, 5088–5102. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhou, X.; Zhang, Z.; Zhu, R.; Lu, M.; Lv, K.; Fang, C.; Ming, Z.; Cheng, Z.; Hu, Y. Mechanism of Bile Acid in Regulating Platelet Function and Thrombotic Diseases. Adv. Sci. 2024, 11, 2401683. [Google Scholar] [CrossRef]
- Von Haehling, S.; Schefold, J.C.; Jankowska, E.A.; Springer, J.; Vazir, A.; Kalra, P.R.; Sandek, A.; Fauler, G.; Stojakovic, T.; Trauner, M.; et al. Ursodeoxycholic acid in patients with chronic heart failure. a double-blind, randomized, placebo-controlled, crossover trial. J. Am. Coll. Cardiol. 2012, 59, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, J.; Zhou, X.; Dai, C.; Kong, M.; Xie, L.; Liu, C.; Liu, Y.; Li, D.; Ma, X.; et al. Ketogenic Diet-Induced Bile Acids Protect against Obesity through Reduced Calorie Absorption. Nat. Metab. 2024, 6, 1397–1414. [Google Scholar] [CrossRef]
- Liu, T.; Zhao, M.; Zhang, Y.; Xu, R.; Fu, Z.; Jin, T.; Song, J.; Huang, Y.; Wang, M.; Zhao, C. Polysaccharides from Phellinus Linteus Attenuate Type 2 Diabetes Mellitus in Rats via Modulation of Gut Microbiota and Bile Acid Metabolism. Int. J. Biol. Macromol. 2024, 262, 130062. [Google Scholar] [CrossRef]
- Gillard, J.; Clerbaux, L.-A.; Nachit, M.; Sempoux, C.; Staels, B.; Bindels, L.B.; Tailleux, A.; Leclercq, I.A. Bile Acids Contribute to the Development of Non-Alcoholic Steatohepatitis in Mice. JHEP Rep. 2022, 4, 100387. [Google Scholar] [CrossRef]
- Dong, S.; Zhu, M.; Wang, K.; Zhao, X.; Hu, L.; Jing, W.; Lu, H.; Wang, S. Dihydromyricetin Improves DSS-Induced Colitis in Mice via Modulation of Fecal-Bacteria-Related Bile Acid Metabolism. Pharmacol. Res. 2021, 171, 105767. [Google Scholar] [CrossRef]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A Human Gut Microbial Gene Catalogue Established by Metagenomic Sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef]
- Human Microbiome Project Consortium. Structure, Function and Diversity of the Healthy Human Microbiome. Nature 2012, 486, 207–214. [Google Scholar] [CrossRef]
- Eiseman, B.; Silen, W.; Bascom, G.S.; Kauvar, A.J. Fecal Enema as an Adjunct in the Treatment of Pseudomembranous Enterocolitis. Surgery. 1958, 44, 854–859. [Google Scholar] [PubMed]
- Schaedler, R.W.; Costello, R. Association of germfree mice with bacteria isolated from normal mice. J. Exp. Med. 1965, 122, 77–82. [Google Scholar] [CrossRef]
- De Vos, W.M.; Tilg, H.; Van Hul, M.; Cani, P.D. Gut Microbiome and Health: Mechanistic Insights. Gut 2022, 71, 1020–1032. [Google Scholar] [CrossRef]
- de Groot, P.; Nikolic, T.; Pellegrini, S.; Sordi, V.; Imangaliyev, S.; Rampanelli, E.; Hanssen, N.; Attaye, I.; Bakker, G.; Duinkerken, G.; et al. Faecal microbiota transplantation halts progression of human new-onset type 1 diabetes in a randomised controlled trial. Gut 2021, 70, 92–105. [Google Scholar] [CrossRef] [PubMed]
- Cotillard, A.; Kennedy, S.P.; Kong, L.C.; Prifti, E.; Pons, N.; Le Chatelier, E.; Almeida, M.; Quinquis, B.; Levenez, F.; Galleron, N.; et al. Dietary Intervention Impact on Gut Microbial Gene Richness. Nature 2013, 500, 585–588. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An Obesity-Associated Gut Microbiome with Increased Capacity for Energy Harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Aron-Wisnewsky, J.; Warmbrunn, M.V.; Nieuwdorp, M.; Clément, K. Metabolism and Metabolic Disorders and the Microbiome: The Intestinal Microbiota Associated With Obesity, Lipid Metabolism, and Metabolic Health—Pathophysiology and Therapeutic Strategies. Gastroenterology 2021, 160, 573–599. [Google Scholar] [CrossRef]
- Wang, Y. Fecal Microbiota Transplantation Attenuates Escherichia Coli Infected Outgrowth by Modulating the Intestinal Microbiome. Microb. Cell Factories 2023, 22, 30. [Google Scholar] [CrossRef]
- Liu, Y.; Dai, M. Trimethylamine N-Oxide Generated by the Gut Microbiota Is Associated with Vascular Inflammation: New Insights into Atherosclerosis. Mediat. Inflamm. 2020, 2020, 1–15. [Google Scholar] [CrossRef]
- Tomlinson, J.A.P.; Wheeler, D.C. The Role of Trimethylamine N-Oxide as a Mediator of Cardiovascular Complications in Chronic Kidney Disease. Kidney Int. 2017, 92, 809–815. [Google Scholar] [CrossRef]
- Akhtar, M.; Chen, Y.; Ma, Z.; Zhang, X.; Shi, D.; Khan, J.A.; Liu, H. Gut Microbiota-Derived Short Chain Fatty Acids Are Potential Mediators in Gut Inflammation. Anim. Nutr. 2022, 8, 350–360. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zhang, P.; Shen, L.; Niu, L.; Tan, Y.; Chen, L.; Zhao, Y.; Bai, L.; Hao, X.; Li, X.; et al. Short-Chain Fatty Acids and Their Association with Signalling Pathways in Inflammation, Glucose and Lipid Metabolism. Int. J. Mol. Sci. 2020, 21, 6356. [Google Scholar] [CrossRef]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef]
- Seethaler, B.; Nguyen, N.K.; Basrai, M.; Kiechle, M.; Walter, J.; Delzenne, N.M.; Bischoff, S.C. Short-Chain Fatty Acids Are Key Mediators of the Favorable Effects of the Mediterranean Diet on Intestinal Barrier Integrity: Data from the Randomized Controlled LIBRE Trial. Am. J. Clin. Nutr. 2022, 116, 928–942. [Google Scholar] [CrossRef]
- Shapiro, H.; Kolodziejczyk, A.A.; Halstuch, D.; Elinav, E. Bile Acids in Glucose Metabolism in Health and Disease. J. Exp. Med. 2018, 215, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Chávez-Talavera, O.; Tailleux, A.; Lefebvre, P.; Staels, B. Bile Acid Control of Metabolism and Inflammation in Obesity, Type 2 Diabetes, Dyslipidemia, and Nonalcoholic Fatty Liver Disease. Gastroenterology 2017, 152, 1679–1694.e3. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Xie, G.; Jia, W. Bile Acid–Microbiota Crosstalk in Gastrointestinal Inflammation and Carcinogenesis. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 111–128. [Google Scholar] [CrossRef]
- Collins, S.L.; Stine, J.G.; Bisanz, J.E.; Okafor, C.D.; Patterson, A.D. Bile Acids and the Gut Microbiota: Metabolic Interactions and Impacts on Disease. Nat. Rev. Microbiol. 2023, 21, 236–247. [Google Scholar] [CrossRef]
- Nemet, I. A Cardiovascular Disease-Linked Gut Microbial Metabolite Acts via Adrenergic Receptors. Cell 2020, 180, 862–877.e22. [Google Scholar] [CrossRef]
- Zhu, Y.; Dwidar, M.; Nemet, I.; Buffa, J.A.; Sangwan, N.; Li, X.S.; Anderson, J.T.; Romano, K.A.; Fu, X.; Funabashi, M.; et al. Two Distinct Gut Microbial Pathways Contribute to Meta-Organismal Production of Phenylacetylglutamine with Links to Cardiovascular Disease. Cell Host Microbe 2023, 31, 18–32.e9. [Google Scholar] [CrossRef]
- Vanweert, F.; Schrauwen, P.; Phielix, E. Role of Branched-Chain Amino Acid Metabolism in the Pathogenesis of Obesity and Type 2 Diabetes-Related Metabolic Disturbances BCAA Metabolism in Type 2 Diabetes. Nutr. Diabetes 2022, 12, 35. [Google Scholar] [CrossRef]
- Nie, C.; He, T.; Zhang, W.; Zhang, G.; Ma, X. Branched Chain Amino Acids: Beyond Nutrition Metabolism. Int. J. Mol. Sci. 2018, 19, 954. [Google Scholar] [CrossRef] [PubMed]
- Holeček, M. Branched-Chain Amino Acids in Health and Disease: Metabolism, Alterations in Blood Plasma, and as Supplements. Nutr. Metab. 2018, 15, 33. [Google Scholar] [CrossRef]
- Sadok, I.; Jędruchniewicz, K. Dietary Kynurenine Pathway Metabolites—Source, Fate, and Chromatographic Determinations. Int. J. Mol. Sci. 2023, 24, 16304. [Google Scholar] [CrossRef] [PubMed]
- Ala, M. Tryptophan Metabolites Modulate Inflammatory Bowel Disease and Colorectal Cancer by Affecting Immune System. Int. Rev. Immunol. 2022, 41, 326–345. [Google Scholar] [CrossRef] [PubMed]
- Joisten, N.; Ruas, J.L.; Braidy, N.; Guillemin, G.J.; Zimmer, P. The Kynurenine Pathway in Chronic Diseases: A Compensatory Mechanism or a Driving Force? Trends Mol. Med. 2021, 27, 946–954. [Google Scholar] [CrossRef]
- Pathak, S.; Nadar, R.; Kim, S.; Liu, K.; Govindarajulu, M.; Cook, P.; Alexander, C.S.W.; Dhanasekaran, M.; Moore, T. The Influence of Kynurenine Metabolites on Neurodegenerative Pathologies. Int. J. Biol. Macromol. 2024, 25, 853. [Google Scholar] [CrossRef]
- Li, M.; Ding, Y.; Wei, J.; Dong, Y.; Wang, J.; Dai, X.; Yan, J.; Chu, F.; Zhang, K.; Meng, F.; et al. Gut Microbiota Metabolite Indole-3-Acetic Acid Maintains Intestinal Epithelial Homeostasis through Mucin Sulfation. Gut Microbes 2024, 16, 2377576. [Google Scholar] [CrossRef]
- Ji, Y.; Gao, Y.; Chen, H.; Yin, Y.; Zhang, W. Indole-3-Acetic Acid Alleviates Nonalcoholic Fatty Liver Disease in Mice via Attenuation of Hepatic Lipogenesis, and Oxidative and Inflammatory Stress. Nutrients 2019, 11, 2062. [Google Scholar] [CrossRef]
- Murray, C.J.L. The Global Burden of Disease Study at 30 Years. Nat. Med. 2022, 28, 2019–2026. [Google Scholar] [CrossRef]
- Mazur, M. Dietary Strategies for Cardiovascular Disease Risk Factors Prevention. Curr. Probl. Cardiol. 2024, 49, 102746. [Google Scholar] [CrossRef] [PubMed]
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Alonso, A.; Beaton, A.Z.; Bittencourt, M.S.; Boehme, A.K.; Buxton, A.E.; Carson, A.P.; Commodore-Mensah, Y.; et al. Heart Disease and Stroke Statistics—2022 Update: A Report From the American Heart Association. Circulation 2022, 145, e153–e639. [Google Scholar] [CrossRef]
- Rout, A.; Duhan, S.; Umer, M.; Li, M.; Kalra, D. Atherosclerotic Cardiovascular Disease Risk Prediction: Current State-of-the-Art. Heart 2024, 110, 1005–1014. [Google Scholar] [CrossRef]
- Timmis, A.; Group, C.W. European Society of Cardiology: Cardiovascular Disease Statistics 2021. Eur. Heart J. 2022, 43, 716–799. [Google Scholar] [CrossRef]
- Witkowski, M.; Weeks, T.L.; Hazen, S.L. Gut Microbiota and Cardiovascular Disease. Circ. Res. 2020, 127, 553–570. [Google Scholar] [CrossRef] [PubMed]
- Bs, M.C.; Mm, L.L.; Bs, S.L.; Chen, L.; Bs, F.J.; Mm, Y.P.; Lin, Y. Gut Microbiota Changes in Patients with Hypertension: A Systematic Review and Meta-Analysis. J. Clin. Hypertens. 2023, 25, 1053–1068. [Google Scholar] [CrossRef]
- Li, J. Gut Microbiota Dysbiosis Contributes to the Development of Hypertension. Microbiome 2017, 5, 14. [Google Scholar] [CrossRef]
- Santisteban, M.M.; Qi, Y.; Zubcevic, J.; Kim, S.; Yang, T.; Shenoy, V.; Cole-Jeffrey, C.T.; Lobaton, G.O.; Stewart, D.C.; Rubiano, A.; et al. Hypertension-Linked Pathophysiological Alterations in the Gut. Circ. Res. 2017, 120, 312–323. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, E.; Yu, Y.; Wang, B.; Zhang, L.; Lei, R.; Xue, B.; Tian, X.; Niu, J.; Liu, J.; et al. Butyrate Attenuates Cold-Induced Hypertension via Gut Microbiota and Activation of Brown Adipose Tissue. Sci. Total Environ. 2024, 943, 173835. [Google Scholar] [CrossRef]
- Bardhan, P.; Mei, X.; Lai, N.K.; Mell, B.; Tummala, R.; Aryal, S.; Manandhar, I.; Hwang, H.; Jhuma, T.A.; Atluri, R.R.; et al. Salt Responsive Gut Microbiota Induces Sex Specific Blood Pressure Changes. Circ. Res. 2024, 135, 1122–1137. [Google Scholar] [CrossRef]
- Jie, Z.; Xia, H.; Zhong, S.-L.; Feng, Q.; Li, S.; Liang, S.; Zhong, H.; Liu, Z.; Gao, Y.; Zhao, H.; et al. The Gut Microbiome in Atherosclerotic Cardiovascular Disease. Nat. Commun. 2017, 8, 845. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zhou, L.; Wang, Q.; Cao, J.-H.; Chen, Y.; Wang, W.; Zhu, B.-D.; Wei, Z.-H.; Li, R.; Li, C.-Y.; et al. Elevated Branched-Chain Amino Acid Promotes Atherosclerosis Progression by Enhancing Mitochondrial-to-Nuclear H2O2-Disulfide HMGB1 in Macrophages. Redox Biol. 2023, 62, 102696. [Google Scholar] [CrossRef] [PubMed]
- Qiao, S.; Liu, C.; Sun, L.; Wang, T.; Dai, H.; Wang, K.; Bao, L.; Li, H.; Wang, W.; Liu, S.-J.; et al. Gut Parabacteroides Merdae Protects against Cardiovascular Damage by Enhancing Branched-Chain Amino Acid Catabolism. Nat. Metab. 2022, 4, 1271–1286. [Google Scholar] [CrossRef] [PubMed]
- Canyelles, M.; Borràs, C.; Rotllan, N.; Tondo, M.; Escolà-Gil, J.C.; Blanco-Vaca, F. Gut Microbiota-Derived TMAO: A Causal Factor Promoting Atherosclerotic Cardiovascular Disease? Int. J. Mol. Sci. 2023, 24, 1940. [Google Scholar] [CrossRef]
- Wang, Z. Gut Flora Metabolism of Phosphatidylcholine Promotes Cardiovascular Disease. Nature 2011, 472, 57–63. [Google Scholar] [CrossRef]
- Zhu, W.; Gregory, J.C.; Org, E.; Buffa, J.A.; Gupta, N.; Wang, Z.; Li, L.; Fu, X.; Wu, Y.; Mehrabian, M.; et al. Gut Microbial Metabolite TMAO Enhances Platelet Hyperreactivity and Thrombosis Risk. Cell 2016, 165, 111–124. [Google Scholar] [CrossRef]
- Seldin, M.M.; Meng, Y.; Qi, H.; Zhu, W.; Wang, Z.; Hazen, S.L.; Lusis, A.J.; Shih, D.M. Trimethylamine N--Oxide Promotes Vascular Inflammation Through Signaling of Mitogen--Activated Protein Kinase and Nuclear Factor--κB. JAHA 2016, 5, e002767. [Google Scholar] [CrossRef]
- Bertero, E.; Maack, C. Metabolic remodelling in heart failure. Nat. Rev. Cardiol. 2018, 15, 457–470. [Google Scholar] [CrossRef]
- Dai, H.; Hou, T.; Wang, Q.; Hou, Y.; Wang, T.; Zheng, J.; Lin, H.; Zhao, Z.; Li, M.; Wang, S.; et al. Causal Relationships between the Gut Microbiome, Blood Lipids, and Heart Failure: A Mendelian Randomization Analysis. Eur. J. Prev. Cardiol. 2023, 30, 1274–1282. [Google Scholar] [CrossRef]
- Cui, X.; Ye, L.; Li, J.; Jin, L.; Wang, W.; Li, S.; Bao, M.; Wu, S.; Li, L.; Geng, B.; et al. Metagenomic and Metabolomic Analyses Unveil Dysbiosis of Gut Microbiota in Chronic Heart Failure Patients. Sci. Rep. 2018, 8, 635. [Google Scholar] [CrossRef]
- Romano, K.A.; Nemet, I.; Prasad Saha, P.; Haghikia, A.; Li, X.S.; Mohan, M.L.; Lovano, B.; Castel, L.; Witkowski, M.; Buffa, J.A.; et al. Gut Microbiota-Generated Phenylacetylglutamine and Heart Failure. Circ. Heart Fail. 2023, 16, e009972. [Google Scholar] [CrossRef] [PubMed]
- Ilardi, F.; Gargiulo, G.; Schiattarella, G.G.; Giugliano, G.; Paolillo, R.; Menafra, G.; De Angelis, E.; Scudiero, L.; Franzone, A.; Stabile, E.; et al. Effects of Carvedilol Versus Metoprolol on Platelet Aggregation in Patients With Acute Coronary Syndrome: The PLATE-BLOCK Study. Am. J. Cardiol. 2018, 122, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Marques, F.Z.; Nelson, E.; Chu, P.-Y.; Horlock, D.; Fiedler, A.; Ziemann, M.; Tan, J.K.; Kuruppu, S.; Rajapakse, N.W.; El-Osta, A.; et al. High-Fiber Diet and Acetate Supplementation Change the Gut Microbiota and Prevent the Development of Hypertension and Heart Failure in Hypertensive Mice. Circulation 2017, 135, 964–977. [Google Scholar] [CrossRef]
- Jarmukhanov, Z.; Mukhanbetzhanov, N.; Kozhakhmetov, S.; Nurgaziyev, M.; Sailybayeva, A.; Bekbossynova, M.; Kushugulova, A. The Association between the Gut Microbiota Metabolite Trimethylamine N-Oxide and Heart Failure. Front. Microbiol. 2024, 15, 1440241. [Google Scholar] [CrossRef] [PubMed]
- Clemente-Suárez, V.J.; Martín-Rodríguez, A.; Redondo-Flórez, L.; López-Mora, C.; Yáñez-Sepúlveda, R.; Tornero-Aguilera, J.F. New Insights and Potential Therapeutic Interventions in Metabolic Diseases. Int. J. Mol. Sci. 2023, 24, 10672. [Google Scholar] [CrossRef]
- Li, D.; Li, Y.; Yang, S.; Lu, J.; Jin, X.; Wu, M. Diet-gut microbiota-epigenetics in metabolic diseases: From mechanisms to therapeutics. Biomed Pharmacother. 2022, 153, 113290. [Google Scholar] [CrossRef]
- Ballini, A.; Scacco, S.; Boccellino, M.; Santacroce, L.; Arrigoni, R. Microbiota and Obesity: Where Are We Now? Biology 2020, 9, 415. [Google Scholar] [CrossRef]
- Ke, W.; Flay, K.J.; Huang, X.; Hu, X.; Chen, F.; Li, C.; Yang, D.A. Polysaccharides from Platycodon Grandiflorus Attenuates High-Fat Diet Induced Obesity in Mice through Targeting Gut Microbiota. Biomed. Pharmacother. 2023, 166, 115318. [Google Scholar] [CrossRef]
- Zhu, M.; Ouyang, J.; Zhou, F.; Zhao, C.; Zhu, W.; Liu, C.; Huang, P.; Li, J.; Tang, J.; Zhang, Z.; et al. Polysaccharides from Fu Brick Tea Ameliorate Obesity by Modulating Gut Microbiota and Gut Microbiota-Related Short Chain Fatty Acid and Amino Acid Metabolism. J. Nutr. Biochem. 2023, 118, 109356. [Google Scholar] [CrossRef]
- Zhang, X.-Y.; Chen, J.; Yi, K.; Peng, L.; Xie, J.; Gou, X.; Peng, T.; Tang, L. Phlorizin Ameliorates Obesity-Associated Endotoxemia and Insulin Resistance in High-Fat Diet-Fed Mice by Targeting the Gut Microbiota and Intestinal Barrier Integrity. Gut Microbes 2020, 12, 1842990. [Google Scholar] [CrossRef]
- Ma, L.; Ni, Y.; Wang, Z.; Tu, W.; Ni, L.; Zhuge, F.; Zheng, A.; Hu, L.; Zhao, Y.; Zheng, L.; et al. Spermidine Improves Gut Barrier Integrity and Gut Microbiota Function in Diet-Induced Obese Mice. Gut Microbes 2020, 12, 1832857. [Google Scholar] [CrossRef]
- Pluznick, J.L. Gut Microbiota in Renal Physiology: Focus on Short-Chain Fatty Acids and Their Receptors. Kidney Int. 2016, 90, 1191–1198. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, A.; Nakatani, A.; Hasegawa, S.; Irie, J.; Ozawa, K.; Tsujimoto, G.; Suganami, T.; Itoh, H.; Kimura, I. The Short Chain Fatty Acid Receptor GPR43 Regulates Inflammatory Signals in Adipose Tissue M2-Type Macrophages. PLoS ONE 2017, 12, e0179696. [Google Scholar] [CrossRef] [PubMed]
- Kimura, I.; Ozawa, K.; Inoue, D.; Imamura, T.; Kimura, K.; Maeda, T.; Terasawa, K.; Kashihara, D.; Hirano, K.; Tani, T.; et al. The Gut Microbiota Suppresses Insulin-Mediated Fat Accumulation via the Short-Chain Fatty Acid Receptor GPR43. Nat. Commun. 2013, 4, 1829. [Google Scholar] [CrossRef]
- He, Z.; Guo, J.; Zhang, H.; Yu, J.; Zhou, Y.; Wang, Y.; Li, T.; Yan, M.; Li, B.; Chen, Y.; et al. Atractylodes macrocephala Koidz polysaccharide improves glycolipid metabolism disorders through activation of aryl hydrocarbon receptor by gut flora-produced tryptophan metabolites. Int. J. Biol. Macromol. 2023, 253 Pt 4, 126987. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Li, M.; Liu, L.; Li, D.; Zhao, L.; Wu, Z.; Zhou, M.; Jia, L.; Yang, F. Cordyceps Militaris Polysaccharide Alleviates Diabetic Symptoms by Regulating Gut Microbiota against TLR4/NF-κB Pathway. Int. J. Biol. Macromol. 2023, 230, 123241. [Google Scholar] [CrossRef]
- Zhou, W.; Yang, T.; Xu, W.; Huang, Y.; Ran, L.; Yan, Y.; Mi, J.; Lu, L.; Sun, Y.; Zeng, X. The polysaccharides from the fruits of Lycium barbarum L. confer anti-diabetic effect by regulating gut microbiota and intestinal barrier. Carbohydr. Polym. 2022, 291, 119626. [Google Scholar] [CrossRef]
- Baars, D.P.; Fondevila, M.F.; Meijnikman, A.S.; Nieuwdorp, M. The Central Role of the Gut Microbiota in the Pathophysiology and Management of Type 2 Diabetes. Cell Host Microbe 2024, 32, 1280–1300. [Google Scholar] [CrossRef]
- Mandaliya, D.K.; Seshadri, S. Short Chain Fatty Acids, Pancreatic Dysfunction and Type 2 Diabetes. Pancreatology 2019, 19, 280–284. [Google Scholar] [CrossRef]
- Bauer, K.C.; Littlejohn, P.T.; Ayala, V.; Creus-Cuadros, A.; Finlay, B.B. Nonalcoholic Fatty Liver Disease and the Gut-Liver Axis: Exploring an Undernutrition Perspective. Gastroenterology 2022, 162, 1858–1875.e2. [Google Scholar] [CrossRef]
- Eslam, M.; Newsome, P.N.; Sarin, S.K.; Anstee, Q.M.; Targher, G.; Romero-Gomez, M.; Zelber-Sagi, S.; Wai-Sun Wong, V.; Dufour, J.-F.; Schattenberg, J.M.; et al. A New Definition for Metabolic Dysfunction-Associated Fatty Liver Disease: An International Expert Consensus Statement. J. Hepatol. 2020, 73, 202–209. [Google Scholar] [CrossRef]
- Eslam, M.; Sanyal, A.J.; George, J.; Sanyal, A.; Neuschwander-Tetri, B.; Tiribelli, C.; Kleiner, D.E.; Brunt, E.; Bugianesi, E.; Yki-Järvinen, H.; et al. MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology 2020, 158, 1999–2014.e1. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, X.; Lin, J.; Liu, J.; Wang, K.; Nie, Q.; Ye, C.; Sun, L.; Ma, Y.; Qu, R.; et al. A Microbial Metabolite Inhibits the HIF-2α-Ceramide Pathway to Mediate the Beneficial Effects of Time-Restricted Feeding on MASH. Cell Metab. 2024, 36, 1823–1838.e6. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Jia, Y.; Hong, J.; Sun, Q.; Gao, S.; Hu, Y.; Zhao, N.; Zhao, R. Sodium Butyrate Ameliorates High-Fat-Diet-Induced Non-Alcoholic Fatty Liver Disease through Peroxisome Proliferator-Activated Receptor α-Mediated Activation of β Oxidation and Suppression of Inflammation. J. Agric. Food Chem. 2018, 66, 7633–7642. [Google Scholar] [CrossRef]
- Liu, W.; Luo, X.; Tang, J.; Mo, Q.; Zhong, H.; Zhang, H.; Feng, F. A Bridge for Short-Chain Fatty Acids to Affect Inflammatory Bowel Disease, Type 1 Diabetes, and Non-Alcoholic Fatty Liver Disease Positively: By Changing Gut Barrier. Eur. J. Nutr. 2021, 60, 2317–2330. [Google Scholar] [CrossRef] [PubMed]
- Min, B.H.; Devi, S.; Kwon, G.H.; Gupta, H.; Jeong, J.-J.; Sharma, S.P.; Won, S.-M.; Oh, K.-K.; Yoon, S.J.; Park, H.J.; et al. Gut Microbiota-Derived Indole Compounds Attenuate Metabolic Dysfunction-Associated Steatotic Liver Disease by Improving Fat Metabolism and Inflammation. Gut Microbes 2024, 16, 2307568. [Google Scholar] [CrossRef]
- Federici, S.; Kviatcovsky, D.; Valdés-Mas, R.; Elinav, E. Microbiome-Phage Interactions in Inflammatory Bowel Disease. Clin. Microbiol. Infect. 2023, 29, 682–688. [Google Scholar] [CrossRef]
- Bretto, E.; Ribaldone, D.G.; Caviglia, G.P.; Saracco, G.M.; Bugianesi, E.; Frara, S. Inflammatory Bowel Disease: Emerging Therapies and Future Treatment Strategies. Biomedicines 2023, 11, 2249. [Google Scholar] [CrossRef]
- IBDMDB Investigators; Lloyd-Price, J.; Arze, C.; Ananthakrishnan, A.N.; Schirmer, M.; Avila-Pacheco, J.; Poon, T.W.; Andrews, E.; Ajami, N.J.; Bonham, K.S.; et al. Multi-Omics of the Gut Microbial Ecosystem in Inflammatory Bowel Diseases. Nature 2019, 569, 655–662. [Google Scholar] [CrossRef]
- Franzosa, E.A.; Sirota-Madi, A.; Avila-Pacheco, J.; Fornelos, N.; Haiser, H.J.; Reinker, S.; Vatanen, T.; Hall, A.B.; Mallick, H.; McIver, L.J.; et al. Gut Microbiome Structure and Metabolic Activity in Inflammatory Bowel Disease. Nat. Microbiol. 2018, 4, 293–305. [Google Scholar] [CrossRef]
- Yang, M.; Gu, Y.; Li, L.; Liu, T.; Song, X.; Sun, Y.; Cao, X.; Wang, B.; Jiang, K.; Cao, H. Bile Acid–Gut Microbiota Axis in Inflammatory Bowel Disease: From Bench to Bedside. Nutrients 2021, 13, 3143. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Chang, L.; Gao, P.; Li, J.; Lu, X.; Hua, M.; Li, X.; Liu, X.; Lan, Y. Synbiotics containing sea buckthorn polysaccharides ameliorate DSS-induced colitis in mice via regulating Th17/Treg homeostasis through intestinal microbiota and their production of BA metabolites and SCFAs. Int. J. Biol. Macromol. 2024, 276 Pt 1, 133794. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Zhai, Y.; Yu, Y.; Shen, J.; Chu, S.; Focaccia, E.; Tian, W.; Wang, S.; Liu, X.; Yuan, X.; et al. TNF Compromises Intestinal Bile-Acid Tolerance Dictating Colitis Progression and Limited Infliximab Response. Cell Metab. 2024, 36, 2086–2103.e9. [Google Scholar] [CrossRef]
- Ozturk, O.; Celebi, G.; Duman, U.G.; Kupcuk, E.; Uyanik, M.; Sertoglu, E. Short-Chain Fatty Acid Levels in Stools of Patients with Inflammatory Bowel Disease Are Lower than Those in Healthy Subjects. Eur. J. Gastroenterol. Hepatol. 2024, 36, 890–896. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Lin, J.; Zhang, C.; Gao, H.; Lu, H.; Gao, X.; Zhu, R.; Li, Z.; Li, M.; Liu, Z. Microbiota Metabolite Butyrate Constrains Neutrophil Functions and Ameliorates Mucosal Inflammation in Inflammatory Bowel Disease. Gut Microbes 2021, 13, 1968257. [Google Scholar] [CrossRef]
- Chen, G.; Ran, X.; Li, B.; Li, Y.; He, D.; Huang, B.; Fu, S.; Liu, J.; Wang, W. Sodium Butyrate Inhibits Inflammation and Maintains Epithelium Barrier Integrity in a TNBS-Induced Inflammatory Bowel Disease Mice Model. eBioMedicine 2018, 30, 317–325. [Google Scholar] [CrossRef]
- Dupraz, L.; Magniez, A.; Rolhion, N.; Richard, M.L.; Da Costa, G.; Touch, S.; Mayeur, C.; Planchais, J.; Agus, A.; Danne, C.; et al. Gut Microbiota-Derived Short-Chain Fatty Acids Regulate IL-17 Production by Mouse and Human Intestinal Γδ T Cells. Cell Rep. 2021, 36, 109332. [Google Scholar] [CrossRef]
- Wang, S.; Van Schooten, F.-J.; Jin, H.; Jonkers, D.; Godschalk, R. The Involvement of Intestinal Tryptophan Metabolism in Inflammatory Bowel Disease Identified by a Meta-Analysis of the Transcriptome and a Systematic Review of the Metabolome. Nutrients 2023, 15, 2886. [Google Scholar] [CrossRef]
- Pernomian, L.; Duarte-Silva, M.; De Barros Cardoso, C.R. The Aryl Hydrocarbon Receptor (AHR) as a Potential Target for the Control of Intestinal Inflammation: Insights from an Immune and Bacteria Sensor Receptor. Clin. Rev. Allerg. Immunol. 2020, 59, 382–390. [Google Scholar] [CrossRef]
- Xie, L.-W.; Cai, S.; Lu, H.-Y.; Tang, F.-L.; Zhu, R.-Q.; Tian, Y.; Li, M. Microbiota-Derived I3A Protects the Intestine against Radiation Injury by Activating AhR/IL-10/Wnt Signaling and Enhancing the Abundance of Probiotics. Gut Microbes 2024, 16, 2347722. [Google Scholar] [CrossRef]
- Hu, X.; Xiao, W.; Lei, Y.; Green, A.; Lee, X.; Maradana, M.R.; Gao, Y.; Xie, X.; Wang, R.; Chennell, G.; et al. Aryl Hydrocarbon Receptor Utilises Cellular Zinc Signals to Maintain the Gut Epithelial Barrier. Nat. Commun. 2023, 14, 5431. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Liu, K.-Y.; Xiao, W.; Xie, X.; Liang, Q.; Tu, Z.; Yang, L.; Yu, H.; Guo, H.; Huang, S.; et al. Aryl Hydrocarbon Receptor Confers Protection against Macrophage Pyroptosis and Intestinal Inflammation through Regulating Polyamine Biosynthesis. Theranostics 2024, 14, 4218–4239. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Fan, Y.; Zhang, G.; Cai, S.; Ma, Y.; Yang, L.; Wang, Y.; Yu, H.; Qiao, S.; Zeng, X. Microbiota-Derived Indoles Alleviate Intestinal Inflammation and Modulate Microbiome by Microbial Cross-Feeding. Microbiome 2024, 12, 59. [Google Scholar] [CrossRef]
- Jiang, L.; Hao, Y.; Han, D.; Dong, W.; Yang, A.; Sun, Z.; Ge, Y.; Duan, S.; Zhang, X.; Dai, Z. Gut Microbiota Dysbiosis Deteriorates Immunoregulatory Effects of Tryptophan via Colonic Indole and LBP/HTR2B-Mediated Macrophage Function. ISME J. 2024, 18, wrae166. [Google Scholar] [CrossRef]
- Feng, R.; Tian, Z.; Mao, R.; Ma, R.; Luo, W.; Zhao, M.; Li, X.; Liu, Y.; Huang, K.; Xiang, L.; et al. Gut Microbiome-Generated Phenylacetylglutamine from Dietary Protein is Associated with Crohn’s Disease and Exacerbates Colitis in Mouse Model Possibly via Platelet Activation. J. Crohn’s Colitis 2023, 17, 1833–1846. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lin, S.; Wang, L.; Cao, Z.; Zhang, M.; Zhang, Y.; Liu, R.; Liu, J. Versatility of Bacterial Outer Membrane Vesicles in Regulating Intestinal Homeostasis. Sci. Adv. 2023, 9, eade5079. [Google Scholar] [CrossRef]
- Taladrid, D.; de Celis, M.; Belda, I.; Bartolomé, B.; Moreno-Arribas, M.V. Hypertension- and Glycaemia-Lowering Effects of a Grape-Pomace-Derived Seasoning in High- Cardiovascular Risk and Healthy Subjects. Interplay with the Gut Microbiome. Food Funct. 2022, 13, 2068–2082. [Google Scholar] [CrossRef]
- Gomez-Arango, L.F.; Barrett, H.L.; McIntyre, H.D.; Callaway, L.K.; Morrison, M.; Nitert, M.D. Increased Systolic and Diastolic Blood Pressure Is Associated With Altered Gut Microbiota Composition and Butyrate Production in Early Pregnancy. Hypertension 2016, 68, 974–981. [Google Scholar] [CrossRef]
- Dan, X.; Mushi, Z.; Baili, W.; Han, L.; Enqi, W.; Huanhu, Z.; Shuchun, L. Differential Analysis of Hypertension-Associated Intestinal Microbiota. Int. J. Med. Sci. 2019, 16, 872–881. [Google Scholar] [CrossRef]
- Nash, D.B. The Future of Chronic Disease Management. Popul. Health Manag. 2023, 26, S-2–S-3. [Google Scholar] [CrossRef]
- Dale, M.T.; Elkins, M.R. Chronic Disease. J. Physiother. 2021, 67, 84–86. [Google Scholar] [CrossRef] [PubMed]
- Luedde, M.; Winkler, T.; Heinsen, F.A.; Rühlemann, M.C.; Spehlmann, M.E.; Bajrovic, A.; Lieb, W.; Franke, A.; Ott, S.J.; Frey, N. Heart Failure Is Associated with Depletion of Core Intestinal Microbiota. ESC Heart Fail. 2017, 4, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Hibberd, A.A.; Yde, C.C.; Ziegler, M.L.; Honoré, A.H.; Saarinen, M.T.; Lahtinen, S.; Stahl, B.; Jensen, H.M.; Stenman, L.K. Probiotic or Synbiotic Alters the Gut Microbiota and Metabolism in a Randomised Controlled Trial of Weight Management in Overweight Adults. BM 2019, 10, 121–136. [Google Scholar] [CrossRef]
- Crovesy, L.; El-Bacha, T.; Rosado, E.L. Modulation of the Gut Microbiota by Probiotics and Symbiotics Is Associated with Changes in Serum Metabolite Profile Related to a Decrease in Inflammation and Overall Benefits to Metabolic Health: A Double-Blind Randomized Controlled Clinical Trial in Women with Obesity. Food Funct. 2021, 12, 2161–2170. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Cho, J.-Y.; Cho, K.Y. Serum, Urine, and Fecal Metabolome Alterations in the Gut Microbiota in Response to Lifestyle Interventions in Pediatric Obesity: A Non-Randomized Clinical Trial. Nutrients 2023, 15, 2184. [Google Scholar] [CrossRef]
- Liu, G.; Liang, L.; Yu, G.; Li, Q. Pumpkin Polysaccharide Modifies the Gut Microbiota during Alleviation of Type 2 Diabetes in Rats. Int. J. Biol. Macromol. 2018, 115, 711–717. [Google Scholar] [CrossRef]
- Xia, T.; Liu, C.-S.; Hu, Y.-N.; Luo, Z.-Y.; Chen, F.-L.; Yuan, L.-X.; Tan, X.-M. Coix Seed Polysaccharides Alleviate Type 2 Diabetes Mellitus via Gut Microbiota-Derived Short-Chain Fatty Acids Activation of IGF1/PI3K/AKT Signaling. Food Res. Int. 2021, 150, 110717. [Google Scholar] [CrossRef]
- Luo, L.; Zhang, H.; Chen, W.; Zheng, Z.; He, Z.; Wang, H.; Wang, K.; Zhang, Y. Angelica sinensis Polysaccharide Ameliorates Nonalcoholic Fatty Liver Disease via Restoring Estrogen-Related Receptor α Expression in Liver. Phytother. Res. 2023, 37, 5407–5417. [Google Scholar] [CrossRef]
- Wang, X.; Shi, L.; Wang, X.; Feng, Y.; Wang, Y. MDG-1, an Ophiopogon Polysaccharide, Restrains Process of Non-Alcoholic Fatty Liver Disease via Modulating the Gut-Liver Axis. Int. J. Biol. Macromol. 2019, 141, 1013–1021. [Google Scholar] [CrossRef]
- He, C.; Wang, H.; Liao, W.-D.; Peng, C.; Shu, X.; Zhu, X.; Zhu, Z.-H. Characteristics of Mucosa-Associated Gut Microbiota during Treatment in Crohn’s Disease. WJG 2019, 25, 2204–2216. [Google Scholar] [CrossRef]
- Wu, J.; Wei, Z.; Cheng, P.; Qian, C.; Xu, F.; Yang, Y.; Wang, A.; Chen, W.; Sun, Z.; Lu, Y. Rhein Modulates Host Purine Metabolism in Intestine through Gut Microbiota and Ameliorates Experimental Colitis. Theranostics 2020, 10, 10665–10679. [Google Scholar] [CrossRef] [PubMed]
- Henn, M.R.; O’Brien, E.J.; Diao, L.; Feagan, B.G.; Sandborn, W.J.; Huttenhower, C.; Wortman, J.R.; McGovern, B.H.; Wang-Weigand, S.; Lichter, D.I.; et al. A Phase 1b Safety Study of SER-287, a Spore-Based Microbiome Therapeutic, for Active Mild to Moderate Ulcerative Colitis. Gastroenterology 2021, 160, 115–127.e30. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Chen, B.; Duan, Z.; Xia, Z.; Ding, Y.; Chen, T.; Liu, H.; Wang, B.; Yang, B.; Wang, X.; et al. Depression and Anxiety in Patients with Active Ulcerative Colitis: Crosstalk of Gut Microbiota, Metabolomics and Proteomics. Gut Microbes 2021, 13, 1987779. [Google Scholar] [CrossRef]
- Ross, F.C.; Patangia, D.; Grimaud, G.; Lavelle, A.; Dempsey, E.M.; Ross, R.P.; Stanton, C. The interplay between diet and the gut microbiome: Implications for health and disease. Nat. Rev. Microbiol. 2024, 22, 671–686. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, T.S.; Rampelli, S.; Jeffery, I.B.; Santoro, A.; Neto, M.; Capri, M.; Giampieri, E.; Jennings, A.; Candela, M.; Turroni, S.; et al. Mediterranean Diet Intervention Alters the Gut Microbiome in Older People Reducing Frailty and Improving Health Status: The NU-AGE 1-Year Dietary Intervention across Five European Countries. Gut 2020, 69, 1218–1228. [Google Scholar] [CrossRef]
- Rondanelli, M.; Gasparri, C.; Peroni, G.; Faliva, M.A.; Naso, M.; Perna, S.; Bazire, P.; Sajuox, I.; Maugeri, R.; Rigon, C. The Potential Roles of Very Low Calorie, Very Low Calorie Ketogenic Diets and Very Low Carbohydrate Diets on the Gut Microbiota Composition. Front. Endocrinol. 2021, 12, 662591. [Google Scholar] [CrossRef]
- Bock, P.M.; Martins, A.F.; Schaan, B.D. Understanding How Pre- and Probiotics Affect the Gut Microbiome and Metabolic Health. Am. J. Physiol.-Endocrinol. Metab. 2024, 327, E89–E102. [Google Scholar] [CrossRef]
- Moszak, M.; Szulińska, M.; Bogdański, P. You Are What You Eat—The Relationship between Diet, Microbiota, and Metabolic Disorders—A Review. Nutrients 2020, 12, 1096. [Google Scholar] [CrossRef]
- Haque, M.; Kaminsky, L.; Abdulqadir, R.; Engers, J.; Kovtunov, E.; Rawat, M.; Al-Sadi, R.; Ma, T.Y. Lactobacillus acidophilus inhibits the TNF-α-induced increase in intestinal epithelial tight junction permeability via a TLR-2 and PI3K-dependent inhibition of NF-κB activation. Front. Immunol. 2024, 15, 1348010. [Google Scholar] [CrossRef]
- Zhou, J. Programmable Probiotics Modulate Inflammation and Gut Microbiota for Inflammatory Bowel Disease Treatment after Effective Oral Delivery. Nat. Commun. 2022, 13, 3432. [Google Scholar] [CrossRef]
- Swanson, K.S.; Gibson, G.R.; Hutkins, R.; Reimer, R.A.; Reid, G.; Verbeke, K.; Scott, K.P.; Holscher, H.D.; Azad, M.B.; Delzenne, N.M.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Synbiotics. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 687–701. [Google Scholar] [CrossRef]
- Paul, P. The Effect of Microbiome-Modulating Probiotics, Prebiotics and Synbiotics on Glucose Homeostasis in Type 2 Diabetes: A Systematic Review, Meta-Analysis, and Meta-Regression of Clinical Trials. Pharmacol. Res. 2022, 185, 106520. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Jin, W.; Liu, S.; Jiao, Z.; Li, X. Probiotics, Prebiotics, and Postbiotics in Health and Disease. MedComm 2023, 4, e420. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-T.; Yang, P.-C.; Zhang, Y.-F.; Sun, J.-F. Synthesis and Clinical Application of New Drugs Approved by FDA in 2023. Eur. J. Med. Chem. 2024, 265, 116124. [Google Scholar] [CrossRef]
- Carson, M.D.; Warner, A.J.; Geiser, V.L.; Hathaway-Schrader, J.D.; Alekseyenko, A.V.; Marshall, J.; Westwater, C.; Novince, C.M. Prolonged Antibiotic Exposure during Adolescence Dysregulates Liver Metabolism and Promotes Adiposity in Mice. Am. J. Pathol. 2023, 193, 796–812. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tang, J.; Lv, Q.; Tan, Y.; Dong, X.; Liu, H.; Zhao, N.; He, Z.; Kou, Y.; Tan, Y.; et al. Establishment and Resilience of Transplanted Gut Microbiota in Aged Mice. iScience 2022, 25, 103654. [Google Scholar] [CrossRef]
- Schenck, L.P.; Beck, P.L.; MacDonald, J.A. Gastrointestinal dysbiosis and the use of fecal microbial transplantation in Clostridium difficile infection. World J. Gastrointest Pathophysiol. 2015, 6, 169–180. [Google Scholar] [CrossRef]
- Yu, E.W.; Gao, L.; Stastka, P.; Cheney, M.C.; Mahabamunuge, J.; Soto, M.T.; Ford, C.B.; Bryant, J.A.; Henn, M.R.; Hohmann, E.L. Fecal Microbiota Transplantation for the Improvement of Metabolism in Obesity: The FMT-TRIM Double-Blind Placebo-Controlled Pilot Trial. PLoS Med. 2020, 17, e1003051. [Google Scholar] [CrossRef]
- Rasmussen, T.S.; Mentzel, C.M.J.; Kot, W.; Castro-Mejía, J.L.; Zuffa, S.; Swann, J.R.; Hansen, L.H.; Vogensen, F.K.; Hansen, A.K.; Nielsen, D.S. Faecal Virome Transplantation Decreases Symptoms of Type 2 Diabetes and Obesity in a Murine Model. Gut 2020, 69, 2122–2130. [Google Scholar] [CrossRef]
- Wu, G.; Xu, T.; Zhao, N.; Lam, Y.Y.; Ding, X.; Wei, D.; Fan, J.; Shi, Y.; Li, X.; Li, M.; et al. A core microbiome signature as an indicator of health. Cell 2024, 187, 6550–6565.e11. [Google Scholar] [CrossRef]
| Metabolites | Disease | Mechanisms | References |
|---|---|---|---|
| TMAO | Hypertension | Promoting Ang II-induced vasoconstriction and thus Ang II-induced hypertension | [8] |
| Atherosclerosis | Increased expression of CD36 and SRA causes cholesterol to accumulate in cells, promotes macrophage foaming, and accelerates atherosclerotic plaque formation | [9] | |
| Heart failure | Enhancing NADPH oxidase NOX activity and increases reactive oxygen species ROS production, further damaging cardiomyocytes and promoting pathological remodeling | [10] | |
| Obesity | Increased visceral fat | [11] | |
| Type 2 diabetes | Reducing the proportion of beta cells and glucose tolerance in mice with impaired insulin secretion | [12] | |
| Metabolic dysfunction-associated steatotic liver disease | Promotes lipid deposition in HepG2 fatty liver cells, disrupting the structure and function of the intestinal barrier and exacerbating hepatic steatosis | [13] | |
| Inflammatory bowel disease | Involvement in IBD pathogenesis by affecting ATG16L1-induced autophagy and activation of NLRP3 inflammasome | [14] | |
| SCFAs | Hypertension | Regulation of microbiome composition, increased expression of SCFA receptors GPR41 and GPR109A, and restoration of RAS homeostasis in kidney | [15] |
| Atherosclerosis | Reduces inflammation, improves metabolic health, and stabilizes plaque | [16] | |
| Heart failure | Improved left ventricular remodeling in mice | [17] | |
| Obesity | Upregulation of UCP1 (a key protein involved in energy expenditure) expression increases white fat browning and energy expenditure | [18] | |
| Type 2 diabetes | Induction of GLP-1 and PYY secretion increases energy expenditure and maintains glucose homeostasis | [19] | |
| Metabolic dysfunction-associated steatotic liver disease | Inhibition of hepatic FASN and CD36 protein expression regulates hepatic lipid metabolism | [20] | |
| Inflammatory bowel disease | Maintaining intestinal homeostasis, enhancing intestinal barrier function, controlling intestinal inflammation | [21] | |
| BAs | Hypertension | Activation of TGR5 in neurons and microglia attenuates inflammatory responses and oxidative stress, inhibits activated neurons, and attenuates hypertension | [22] |
| Atherosclerosis | Activation of TGR5, reduction in macrophage inflammation and lipid uptake, inhibition of platelet activation | [23] | |
| Heart failure | Reduces pro-inflammatory factors and improves peripheral blood flow | [24] | |
| Obesity | Reduces energy absorption by inhibiting intestinal Car1 expression, leading to weight loss | [25] | |
| Type 2 diabetes | Promote GLP-1 secretion, thus increasing insulin release and lowering blood glucose | [26] | |
| Metabolic dysfunction-associated steatotic liver disease | Increases TGR5 and FXR signaling, improves metabolic disorders, prevents steatosis and hepatocyte ballooning, and reduces macrophage infiltration | [27] | |
| Inflammatory bowel disease | Activation of intestinal epithelial FXR/TGR5-related signaling pathway to inhibit inflammatory response and repair intestinal barrier integrity | [28] |
| Disease | Model | Sample Size | Gut Microbiota | Metabolites | References |
|---|---|---|---|---|---|
| Hypertension | Human | n = 29 | Escherichia/Shigella ↑ Tyzerella 4, Gordonibacter and Fournierella | Acetic, propionic, and butyric acids ↓ | [127] |
| Hypertension | Human | n = 205 | Odoribacter, Clostridiaceae ↓ | Butyrate ↓ | [128] |
| Hypertension | Human | n = 129 | Parabacteroides, Desulfovibrio, Christensenella, Alistipes ↑ Prevotella, Lactobacillus ↓ | SCFAs ↓, TMAO ↑ | [129] |
| Atherosclerosis | Human | n = 76 | Prevotella copri ↑ | TMAO ↑ | [130] |
| Atherosclerosis | Human | n = 405 | Enterobacteriaceae and Streptococcus spp. ↑ Bacteroides and Prevotella ↓ | / | [71] |
| Atherosclerosis | Female and male C57BL/6J mice | n = 342 | Roseburia ↓ | Butyrate ↓ | [131] |
| Heart Failure | Human | n = 94 | Faecalibacterium prausnitzii ↓ and Ruminococcus gnavus ↑ | Butyrate ↓ TMAO ↑ | [80] |
| Heart Failure | Human | n = 24 | Eubacterium rectale and Dorea longicatena ↓ | Butyrate, acetate ↓ | [124] |
| Heart Failure | Human | n = 20 | Blautia, Collinsella, uncl. Erysipelotrichaceae and uncl. Ruminococcaceae ↓ | / | [132] |
| Obesity | Human | n = 134 | Lactobacillus, Akkermansia, Christensenellaceae, Methanobrevibacter ↓ Paraprevotella ↑ | Bile acids, glycocholic acid, glycoursodeoxycholic acid, taurohyodeoxycholic acid, and tauroursodeoxycholic acid ↑ | [133] |
| Obesity | Human | n = 32 | Bacteroidetes ↑ Firmicutes, Verrucomicrobia ↓ | Arginine, glutamine, 2-oxoisovalerate, pyruvate, alanine ↑; citrate, BCAA ↓ | [134] |
| Obesity | Human | n = 72 | Eubacterium hallii, Ruminococcus gnavus groups, and Dorea ↑ | L-isoleucine, uric acid ↑Taurodeoxycholic, tauromuricholic α + β acid, myristic acid ↓ | [135] |
| Type 2 Diabetes | Male Wistar rats | n = 30 | Bacteroidetes, Prevotella, Deltaproteobacteria, Oscillospira, Veillonellaceae, Phascolarctobacterium, Sutterella, Bilophila ↓ | Acetic acid, propionic acid, butyric acid, isobutyric acid, valeric acid, isovaleric acid ↓ | [136] |
| Type 2 Diabetes | Male C57BL/6J mice | n = 48 | Bacteroidetes ↓ Firmicutes ↓ Lactobacillus, Akkermansia, Bacteroides, Bifidobacterium ↓ Helicobacter ↑ | Acetic acid, propionic acid, butyric acid, isobutyric acid, valeric acid, isovaleric acid ↓ | [137] |
| Metabolic Dysfunction-Associated Steatotic Liver Disease | Female BALB/c mice | n = 24 | Firmicutes, Bacteroidetes, Proteobacteria ↓ Akkermansia muciniphila, Clostridium leptum, Ruminococcus gnavus ↓ | Propionic acid, butyric acid ↓ | [138] |
| Metabolic Dysfunction-Associated Steatotic Liver Disease | Male C57BL/6J mice | n = 40 | Alistipes, Ruminiclostridium, Rikenella ↓ Lactococcus, Enterorhabdus, Turicibacter, Clostridium-sensu-stricto-1, Tyzzerella, Oscillibacter ↑ | Acetic acid, propionic acid, butyric acid, isobutyric acid, valeric acid, isovaleric acid ↓ | [139] |
| Metabolic Dysfunction-Associated Steatotic Liver Disease | Human | n = 61 | Bacteroidetes ↓, Bifidobacterium, Firmicutes ↑ | Cholic acid, chenodeoxycholic acid ↑ | [106] |
| Crohn’s Disease | Human | n = 220 | Bifidobacterium breve, Clostridium symbiosum ↑ Roseburia hominis, Dorea formicigenerans, Ruminococcus obeum ↓ | Cholic acid, deoxycholic acid ↑ | [109] |
| Crohn’s Disease | Human | n = 15 | Anaerostipes, Roseburia, Ruminococcus, Lactobacillus ↓ | Acetic acid, propionic acid, butyric acid ↓ | [140] |
| Crohn’s Disease | Male C57BL/6J mice | / | Lactobacillus ↓ | purine ↑ uric acid ↓ | [141] |
| Ulcerative Colitis | Human | n = 58 | Enterobacteriaceae, Veillonella, Streptococcus, and Bacteroides ↑ | Hexanoate, butyrate/propionate, LCA and DCA ↓ UDCA ↑ | [142] |
| Ulcerative Colitis | Human | n = 240 | Lactobacillales, Sellimonas, Streptococcus ↑ Prevotella_9, Lachnospira ↓ | Glycocholic acid, glycochenodeoxycholic acid ↑ | [143] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.; Wang, L.; Ou, J.; Peng, D.; Zhang, Y.; Chen, W.; Wang, Y. Gut Microbiota Metabolites and Chronic Diseases: Interactions, Mechanisms, and Therapeutic Strategies. Int. J. Mol. Sci. 2025, 26, 3752. https://doi.org/10.3390/ijms26083752
Liu W, Wang L, Ou J, Peng D, Zhang Y, Chen W, Wang Y. Gut Microbiota Metabolites and Chronic Diseases: Interactions, Mechanisms, and Therapeutic Strategies. International Journal of Molecular Sciences. 2025; 26(8):3752. https://doi.org/10.3390/ijms26083752
Chicago/Turabian StyleLiu, Wenwen, Lei Wang, Jinmei Ou, Daiyin Peng, Yue Zhang, Weidong Chen, and Yanyan Wang. 2025. "Gut Microbiota Metabolites and Chronic Diseases: Interactions, Mechanisms, and Therapeutic Strategies" International Journal of Molecular Sciences 26, no. 8: 3752. https://doi.org/10.3390/ijms26083752
APA StyleLiu, W., Wang, L., Ou, J., Peng, D., Zhang, Y., Chen, W., & Wang, Y. (2025). Gut Microbiota Metabolites and Chronic Diseases: Interactions, Mechanisms, and Therapeutic Strategies. International Journal of Molecular Sciences, 26(8), 3752. https://doi.org/10.3390/ijms26083752





