Bacteria-Mediated Anomalous Rho GTPase Activation Alters Sperm Structure and Provokes Premature Capacitation Events: A Possible Mechanism of Infertility
Abstract
1. Introduction
2. Results
2.1. RhoA GTPase Activity
2.2. Viability and Mitochondrial Membrane Potential
2.3. Sperm Motility
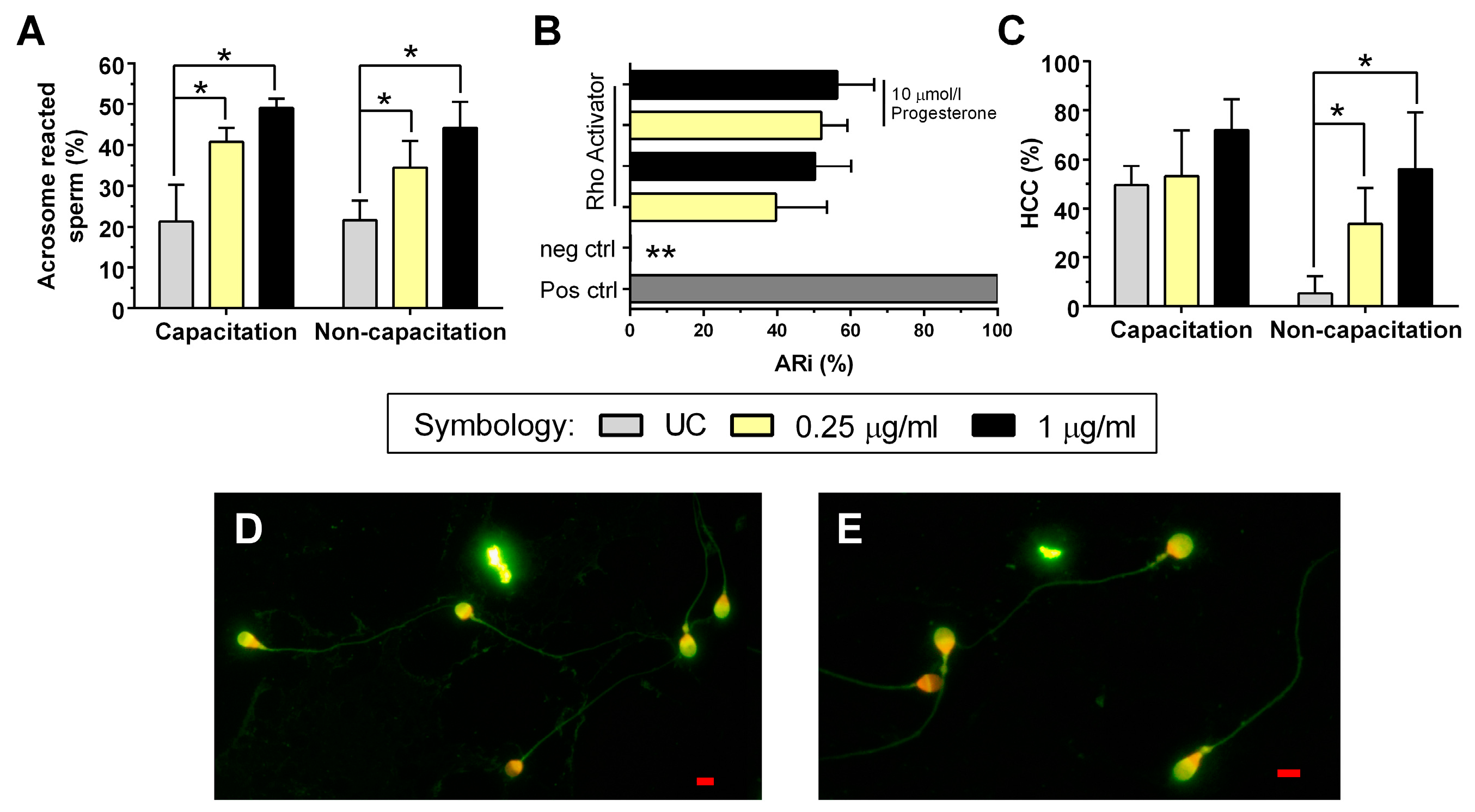
2.4. Acrosome Reaction
2.5. Intracellular Calcium
2.6. Sperm Morphology
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Sperm Collection and Selection
4.3. Experimental Conditions and Activation of Rho GTPases
4.4. Experimental Design
4.4.1. Measuring RhoA GTPase Activity
4.4.2. Evaluation of Viability and Mitochondrial Membrane Potential
4.4.3. Measuring of Sperm Motility
4.4.4. Evaluation of the Acrosome Reaction (AR) and Calculation of the AR Index (ARi)
4.4.5. Measurement of Intracellular Calcium
4.4.6. Sperm Morphology Analysis
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization; World Bank. World Report on Disability, 1st ed.; WHO Press: Geneva, Switzerland, 2011; p. 325. [Google Scholar]
- Agarwal, A.; Mulgund, A.; Hamada, A.; Chyatte, M.R. A unique view on male infertility around the globe. Reprod. Biol. Endocrinol. 2015, 13, 37. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Baskaran, S.; Parekh, N.; Cho, C.-L.; Henkel, R.; Vij, S.; Arafa, M.; Selvam, M.K.P.; Shah, R. Male infertility. Lancet 2021, 397, 319–333. [Google Scholar] [CrossRef]
- Dohle, G.R. Inflammatory-associated obstructions of the male reproductive tract. Andrologia 2003, 35, 321–324. [Google Scholar] [CrossRef] [PubMed]
- Gallegos, G.; Ramos, B.; Santiso, R.; Goyanes, V.; Gosálvez, J.; Fernández, J.L. Sperm DNA fragmentation in infertile men with genitourinary infection by Chlamydia trachomatis and Mycoplasma. Fertil. Steril. 2008, 90, 328–334. [Google Scholar] [CrossRef]
- Flores-Mireles, A.L.; Walker, J.N.; Caparon, M.; Hultgren, S.J. Urinary tract infections: Epidemiology, mechanisms of infection and treatment options. Nat. Rev. Microbiol. 2015, 13, 269–284. [Google Scholar] [CrossRef] [PubMed]
- Rusz, A.; Pilatz, A.; Wagenlehner, F.; Linn, T.; Diemer, T.; Schuppe, H.C.; Lohmeyer, J.; Hossain, H.; Weidner, W. Influence of urogenital infections and inflammation on semen quality and male fertility. World J. Urol. 2012, 30, 23–30. [Google Scholar] [CrossRef]
- Boguen, R.; Treulen, F.; Uribe, P.; Villegas, J.V. Ability of Escherichia coli to produce hemolysis leads to a greater pathogenic effect on human sperm. Fertil. Steril. 2015, 103, 1155–1161. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, J.T.; Riese, M.J.; Aktories, K. Bacterial toxins that modify the actin cytoskeleton. Annu. Rev. Cell Dev. Biol. 2002, 18, 315–344. [Google Scholar] [CrossRef] [PubMed]
- Thalmann, J.; Janik, K.; May, M.; Sommer, K.; Ebeling, J.; Hofmann, F.; Genth, H.; Klos, A. Actin Re-Organization Induced by Chlamydia trachomatis Serovar D—Evidence for a Critical Role of the Effector Protein CT166 Targeting Rac. PLoS ONE 2010, 5, e9887. [Google Scholar] [CrossRef] [PubMed]
- Hodge, R.G.; Ridley, A.J. Regulating Rho GTPases and their regulators. Nat. Rev. Mol. Cell Biol. 2016, 17, 496–510. [Google Scholar] [CrossRef] [PubMed]
- Feltri, M.L.; Suter, U.; Relvas, J.B. The function of RhoGTPases in axon ensheathment and myelination. Glia 2008, 56, 1508–1517. [Google Scholar] [CrossRef] [PubMed]
- Lemichez, E.; Aktories, K. Hijacking of Rho GTPases during bacterial infection. Exp. Cell Res. 2013, 319, 2329–2336. [Google Scholar] [CrossRef] [PubMed]
- Ducummon, C.C.; Berger, T. Localization of the Rho GTPases and some Rho effector proteins in the sperm of several mammalian species. Zygote 2006, 14, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Ramírez, D.; Salgado-Lucio, M.L.; Roa-Espitia, A.L.; Fierro, R.; González-Márquez, H.; Cordero-Martínez, J.; Hernández-González, E.O. Rac1 is necessary for capacitation and acrosome reaction in guinea pig spermatozoa. J. Cell. Biochem. 2020, 121, 2864–2876. [Google Scholar] [CrossRef] [PubMed]
- Mortimer, D. The functional anatomy of the human spermatozoon: Relating ultrastructure and function. Mol. Hum. Reprod. 2018, 24, 567–592. [Google Scholar] [CrossRef] [PubMed]
- Breitbart, H.; Cohen, G.; Rubinstein, S. Role of actin cytoskeleton in mammalian sperm capacitation and the acrosome reaction. Reproduction 2005, 129, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Breitbart H, Finkelstein M Actin cytoskeleton and sperm function. Biochem. Biophys. Res. Commun. 2018, 506, 372–377. [CrossRef] [PubMed]
- Yanagimachi, R. Mammalian fertilization. In The Physiology of Reproduction, 2nd ed.; Knobil, E., Neill, J., Eds.; Raven Press: New York, NY, USA, 1994; pp. 189–317. [Google Scholar]
- Tan, W.; Pang, Y.; Tubbs, C.; Thomas, P. Induction of sperm hypermotility through membrane progestin receptor alpha (mPRα): A teleost model of rapid, multifaceted, nongenomic progestin signaling. Gen. Comp. Endocrinol. 2019, 279, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Carlson, A.E.; Westenbroek, R.E.; Quill, T.; Ren, D.; Clapham, D.E.; Hille, B.; Garbers, D.L.; Babcock, D.F. CatSper1 required for evoked Ca 2+ entry and control of flagellar function in sperm. Proc. Natl. Acad. Sci. USA 2003, 100, 14864–14868. [Google Scholar] [CrossRef]
- Prajapati, P.; Kane, S.; McBrinn, R.C.; Dean, M.S.; da Silva, S.J.M.; Brown, S.G. Elevated and Sustained Intracellular Calcium Signalling Is Necessary for Efficacious Induction of the Human Sperm Acrosome Reaction. Int. J. Mol. Sci. 2022, 23, 11253. [Google Scholar] [CrossRef]
- Vodstrcil, L.A.; McIver, R.; Huston, W.M.; Tabrizi, S.N.; Timms, P.; Hocking, J.S. The Epidemiology of Chlamydia trachomatis Organism Load During Genital Infection: A Systematic Review. J. Infect. Dis. 2015, 211, 1628–1645. [Google Scholar] [CrossRef]
- Reyes-Miguel, T.; Roa-Espitia, A.L.; Baltiérrez-Hoyos, R.; O Hernández-González, E. CDC42 drives RHOA activity and actin polymerization during capacitation. Reproduction 2020, 160, 393–404. [Google Scholar] [CrossRef] [PubMed]
- Amaral, A.; Herrmann, B.G. RAC1 controls progressive movement and competitiveness of mammalian spermatozoa. PLoS Genet. 2021, 17, e1009308. [Google Scholar] [CrossRef] [PubMed]
- Nahacka, Z.; Novak, J.; Zobalova, R.; Neuzil, J. Miro proteins and their role in mitochondrial transfer in cancer and beyond. Front. Cell Dev. Biol. 2022, 10, 937753. [Google Scholar] [CrossRef] [PubMed]
- Fujinoki, M, Non-genomic regulation of mammalian sperm hyperactivation. Reprod. Med. Biol. 2009, 8, 47–52. [CrossRef]
- Pablo, J.L.; DeCaen, P.G.; Clapham, D.E. Progress in ciliary ion channel physiology. J. Gen. Physiol. 2017, 149, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Vyklicka, L.; Lishko, P.V. Dissecting the signaling pathways involved in the function of sperm flagellum. Curr. Opin. Cell Biol. 2020, 63, 154–161. [Google Scholar] [CrossRef] [PubMed]
- El-Mulla, K.F.; Kohn, F.M.; Dandal, M.; El Beheiry, A.H.; Schiefer, H.G.; Weidner, W.; Schill, W.B. In vitro effect of Escherichia coli on human sperm acrosome reaction. Arch. Androl. 1996, 37, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Suhaiman, L.; Altamirano, K.N.; Morales, A.; Belmonte, S.A. Different Approaches to Record Human Sperm Exocytosis. Methods Mol. Biol. 2021, 2233, 139–168. [Google Scholar] [CrossRef] [PubMed]
- Bafor, E.E.; Rowan, E.G.; Edrada-Ebel, R. Metabolomics-Coupled Functional Pharmacology of Chlorophyll Compounds Isolated From the Leaves of Ficus Exasperata Vahl (Moraceae) Provides Novel Pathways on Myometrial Activity. Reprod. Sci. 2018, 25, 923–937. [Google Scholar] [CrossRef]
- Barón, L.; Fara, K.; Zapata-Carmona, H.; Zuñiga, L.; Kong, M.; Signorelli, J.; Díaz, E.S.; Morales, P. Participation of protein kinases and phosphatases in the progesterone-induced acrosome reaction and calcium influx in human spermatozoa. Andrology 2016, 4, 1073–1083. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, M.; Etkovitz, N.; Breitbart, H. Role and regulation of sperm gelsolin prior to fertilization. J. Biol. Chem. 2010, 285, 39702–39709. [Google Scholar] [CrossRef] [PubMed]
- Diemer, T.; Huwe, P.; Ludwig, M.; Schroeder-Printzen, I.; Michelmann, H.W.; Schiefer, H.G.; Weidner, W. Influence of autogenous leucocytes and Escherichia coli on sperm motility parameters in vitro. Andrologia 2003, 35, 100–105. [Google Scholar] [CrossRef]
- Boisen, I.M.; Rehfeld, A.; Mos, I.; Poulsen, N.N.; Nielsen, J.E.; Schwarz, P.; Rejnmark, L.; Dissing, S.; Bach-Mortensen, P.; Juul, A.; et al. The calcium-sensing receptor is essential for calcium and bicarbonate sensitivity in human spermatozoa. J. Clin. Endocrinol. Metab. 2021, 106, 1775–1792. [Google Scholar] [CrossRef]
- Mundt, N.; Spehr, M.; Lishko, P.V. TRPV4 is the temperature-sensitive ion channel of human sperm. Elife 2018, 7, e35853. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Cheng, Y.; Wang, Z.; Zhou, J.; Jia, Y.; He, X.; Zhao, L.; Dong, Y.; Fan, Y.; Yang, X.; et al. Calcium and TRPV4 promote metastasis by regulating cytoskeleton through the RhoA/ROCK1 pathway in endometrial cancer. Cell Death Dis. 2020, 11, 1009. [Google Scholar] [CrossRef] [PubMed]
- Komiya, A.; Watanabe, A.; Kawauchi, Y.; Fuse, H. Sperm with large nuclear vacuoles and semen quality in the evaluation of male infertility. Syst. Biol. Reprod. Med. 2013, 59, 13–20. [Google Scholar] [CrossRef]
- Neyer, A.; Vanderzwalmen, P.; Bach, M.; Stecher, A.; Spitzer, D.; Zech, N. Sperm head vacuoles are not affected by in-vitro conditions, as analysed by a system of sperm-microcapture channels. Reprod. Biomed. Online 2013, 26, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Berkovitz, A.; Eltes, F.; Ellenbogen, A.; Peer, S.; Feldberg, D.; Bartoov, B. Does the presence of nuclear vacuoles in human sperm selected for ICSI affect pregnancy outcome? Hum. Reprod. 2006, 21, 1787–1790. [Google Scholar] [CrossRef] [PubMed]
- A Quintero, C.; Tudela, J.G.; Damiani, M.T. Rho GTPases as pathogen targets: Focus on curable sexually transmitted infections. Small GTPases 2015, 6, 108–118. [Google Scholar] [CrossRef]
- World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen, 6th ed.; WHO: Geneva, Switzerland, 2021; p. 276. [Google Scholar]
- Nobes, C.D.; Hall, A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell 1995, 81, 53–62. [Google Scholar] [CrossRef]
- Gasic, I.; Mitchison, T.J. Autoregulation and repair in microtubule homeostasis. Curr. Opin. Cell Biol. 2019, 56, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Itach, S.B.-S.; Finklestein, M.; Etkovitz, N.; Breitbart, H. Hyper-activated motility in sperm capacitation is mediated by phospholipase D-dependent actin polymerization. Dev. Biol. 2012, 362, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Surrey, E.S. Impact of intramural leiomyomata on in-vitro fertilization-embryo transfer cycle outcome. Curr. Opin. Obstet. Gynecol. 2003, 15, 239–242. [Google Scholar] [CrossRef] [PubMed]
- Strünker, T.; Goodwin, N.; Brenker, C.; Kashikar, N.D.; Weyand, I.; Seifert, R.; Kaupp, U.B. The CatSper channel mediates progesterone-induced Ca2+ influx in human sperm. Nature 2011, 471, 382–386. [Google Scholar] [CrossRef]
- Silvestroni, L.; Mantovani, A.; Palleschi, S. The partial head decondensation test is a new, quick method to assess acrosome status in human spermatozoa. Fertil. Steril. 2004, 81, 1007–1012. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, J.A.; Schulz, M.A.; Sánchez, R.; Villegas, J.V. Integrity of mitochondrial membrane potential reflects human sperm quality. Andrologia 2009, 41, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Uribe, P.; Meriño, J.; Bravo, A.; Zambrano, F.; Schulz, M.; Villegas, J.V.; Sánchez, R. Antioxidant effects of penicillamine against in vitro-induced oxidative stress in human spermatozoa. Andrologia 2020, 52, e13553. [Google Scholar] [CrossRef]


| Capacitation Condition | Non-Capacitation Condition | |||||
|---|---|---|---|---|---|---|
| Abnormal Morphology | RAII | RAII | ||||
| UC (%) | 0.25 μg/mL (%) | 1 μg/mL (%) | UC (%) | 0.25 μg/mL (%) | 1 μg/mL (%) | |
| Vacuolated head | 0.0 ± 0.0 | 10.5 ± 3.4 a | 6.0 ± 6.4 a | 0.0 ± 0.0 | 14.3 ± 4.9 a | 13.0 ± 5.9 a |
| Cut neck | 0.0 ± 0.0 | 3.8 ± 7.5 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.8 ± 1.5 | 0.0 ± 0.0 |
| Bent neck | 10.8 ± 15.6 | 2.3 ± 4.5 | 9.8 ± 11.3 | 14.8 ± 23.9 | 6.5 ± 6.0 | 2.0 ± 2.4 |
| Cut tail | 10.0 ± 9.1 | 8.8 ± 4.9 | 5.5 ± 3.9 | 1.8 ± 3.5 | 11.0 ± 8.9 a | 5.5 ± 6.6 |
| Coiled tail | 42.5 ± 19.7 | 50.0 ± 13.5 | 36.0 ± 26.2 | 36.3 ± 32.0 | 36.8 ± 24.0 | 37.8 ± 12.6 |
| Bent tail | 1.8 ± 3.5 | 7.0 ± 12.1 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.8 ± 1.5 |
| Z-shaped tail | 9.3 ± 10.8 | 0.0 ± 0.0 | 7.8 ± 9.7 | 6.8 ± 9.4 | 15.8 ± 18.6 | 32.0 ± 34.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivera, B.; Aroca, C.; González, B.; Guzmán, N.; Letelier, P.; Uribe, P.; Fornés, M.; Villegas, J.V.; Boguen, R. Bacteria-Mediated Anomalous Rho GTPase Activation Alters Sperm Structure and Provokes Premature Capacitation Events: A Possible Mechanism of Infertility. Int. J. Mol. Sci. 2025, 26, 3783. https://doi.org/10.3390/ijms26083783
Rivera B, Aroca C, González B, Guzmán N, Letelier P, Uribe P, Fornés M, Villegas JV, Boguen R. Bacteria-Mediated Anomalous Rho GTPase Activation Alters Sperm Structure and Provokes Premature Capacitation Events: A Possible Mechanism of Infertility. International Journal of Molecular Sciences. 2025; 26(8):3783. https://doi.org/10.3390/ijms26083783
Chicago/Turabian StyleRivera, Bárbara, Claudia Aroca, Braian González, Neftalí Guzmán, Pablo Letelier, Pamela Uribe, Miguel Fornés, Juana Valentina Villegas, and Rodrigo Boguen. 2025. "Bacteria-Mediated Anomalous Rho GTPase Activation Alters Sperm Structure and Provokes Premature Capacitation Events: A Possible Mechanism of Infertility" International Journal of Molecular Sciences 26, no. 8: 3783. https://doi.org/10.3390/ijms26083783
APA StyleRivera, B., Aroca, C., González, B., Guzmán, N., Letelier, P., Uribe, P., Fornés, M., Villegas, J. V., & Boguen, R. (2025). Bacteria-Mediated Anomalous Rho GTPase Activation Alters Sperm Structure and Provokes Premature Capacitation Events: A Possible Mechanism of Infertility. International Journal of Molecular Sciences, 26(8), 3783. https://doi.org/10.3390/ijms26083783







