Diastolic Dysfunction with Vascular Deficits in HIV-1-Infected Female Humanized Mice Treated with Antiretroviral Drugs
Abstract
1. Introduction
2. Results
2.1. Characterization of Humanized Mice Used in Study
2.2. Echocardiographic Analyses
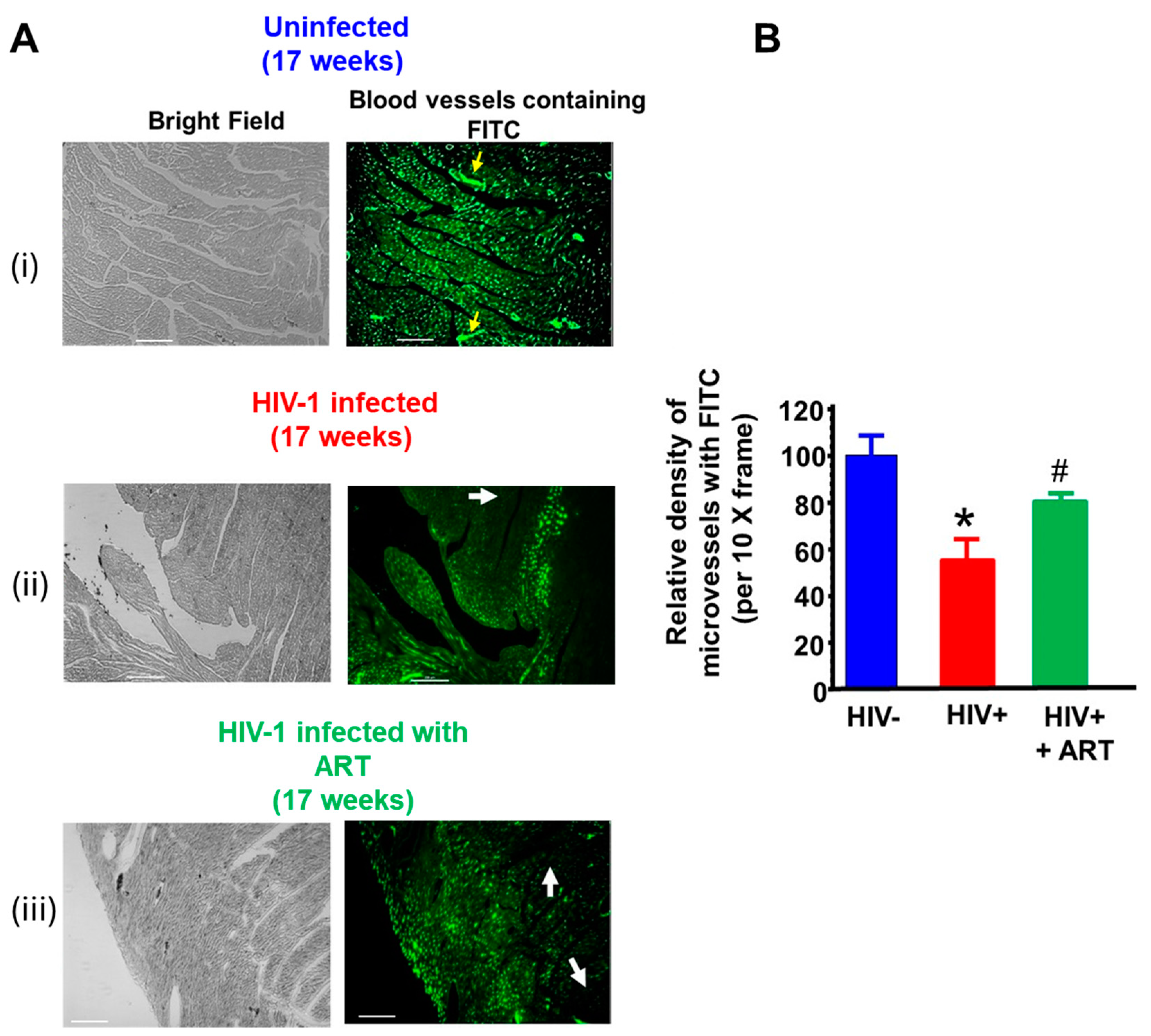
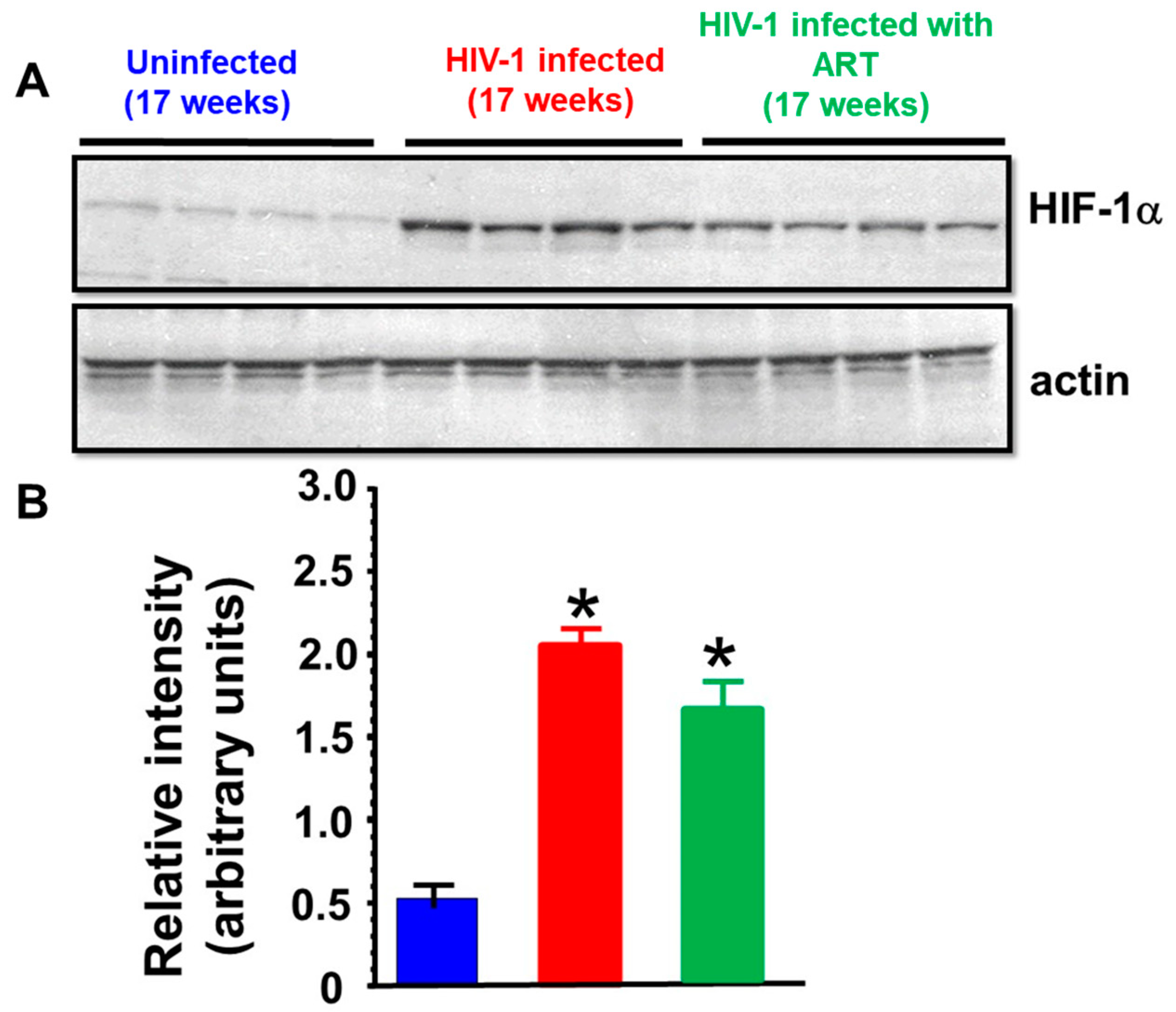
2.3. Histopathological Analyses
3. Discussion
Limitations
4. Material and Methods
4.1. Ethics Statement
4.2. Antibodies and Reagents
4.3. Cell Culture
4.4. Generation of Hu-NSG (Humanized) Mice
4.5. HIV-1 Infection
4.6. Antiretroviral Drug Treatment
4.7. Echocardiography
4.8. Speckle Tracking
4.9. Photoacoustic Imaging
4.10. Histopathological Analyses
4.11. Treatment of H9C2 Cells with Antiretroviral Drugs
4.12. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- May, M.T.; Ingle, S.M. Life expectancy of HIV-positive adults: A review. Sex. Health 2011, 8, 526–533. [Google Scholar] [CrossRef]
- Afzal, M.; Agarwal, S.; Elshaikh, R.H.; Babker, A.M.A.; Osman, E.A.I.; Choudhary, R.K.; Jaiswal, S.; Zahir, F.; Prabhakar, P.K.; Abbas, A.M.; et al. Innovative Diagnostic Approaches and Challenges in the Management of HIV: Bridging Basic Science and Clinical Practice. Life 2025, 15, 209. [Google Scholar] [CrossRef]
- Barbaro, G.; Barbarini, G.; Di Lorenzo, G.; The Gruppo Italiano Per lo Studio Cardiologico dei Pazienti Affetti da AIDS. Early impairment of systolic and diastolic function in asymptomatic HIV-positive patients: A multicenter echocardiographic and echo-Doppler study. AIDS Res. Hum. Retroviruses 1996, 12, 1559–1563. [Google Scholar] [CrossRef]
- Coudray, N.; de Zuttere, D.; Force, G.; de Ribes, D.C.; Pourny, J.C.; Antony, I.; Lecarpentier, Y.; Chemla, D. Left ventricular diastolic function in asymptomatic and symptomatic human immunodeficiency virus carriers: An echocardiographic study. Eur. Heart J. 1995, 16, 61–67. [Google Scholar] [CrossRef]
- Longo-Mbenza, B.; Seghers, L.V.; Vita, E.K.; Tonduangu, K.; Bayekula, M. Assessment of ventricular diastolic function in AIDS patients from Congo: A Doppler echocardiographic study. Heart 1998, 80, 184–189. [Google Scholar] [CrossRef]
- Martinez-Garcia, T.; Sobrino, J.M.; Pujol, E.; Galvez, J.; Benitez, E.; Giron-Gonzalez, J.A. Ventricular mass and diastolic function in patients infected by the human immunodeficiency virus. Heart 2000, 84, 620–624. [Google Scholar] [CrossRef] [PubMed]
- Butler, J.; Kalogeropoulos, A.P.; Anstrom, K.J.; Hsue, P.Y.; Kim, R.J.; Scherzer, R.; Shah, S.J.; Shah, S.H.; Velazquez, E.J.; Hernandez, A.F.; et al. Diastolic Dysfunction in Individuals With Human Immunodeficiency Virus Infection: Literature Review, Rationale and Design of the Characterizing Heart Function on Antiretroviral Therapy (CHART) Study. J. Card. Fail. 2018, 24, 255–265. [Google Scholar] [CrossRef]
- Okeke, N.L.; Alenezi, F.; Bloomfield, G.S.; Dunning, A.; Clement, M.E.; Shah, S.H.; Naggie, S.; Velazquez, E.J. Determinants of Left Ventricular Hypertrophy and Diastolic Dysfunction in an HIV Clinical Cohort. J. Card. Fail. 2018, 24, 496–503. [Google Scholar] [CrossRef]
- Fontes-Carvalho, R.; Mancio, J.; Marcos, A.; Sampaio, F.; Mota, M.; Rocha Goncalves, F.; Gama, V.; Azevedo, A.; Leite-Moreira, A. HIV patients have impaired diastolic function that is not aggravated by anti-retroviral treatment. Cardiovasc. Drugs Ther. 2015, 29, 31–39. [Google Scholar] [CrossRef]
- Arodiwe, I.O.; Eke, C.B. Assessment of left ventricular diastolic function in children with HIV/AIDS attending a tertiary health Facility in Enugu, Nigeria: A Doppler echocardiographic study. BMC Pediatr. 2022, 22, 652. [Google Scholar] [CrossRef]
- Janjua, S.A.; Triant, V.A.; Addison, D.; Szilveszter, B.; Regan, S.; Staziaki, P.V.; Grinspoon, S.A.; Hoffmann, U.; Zanni, M.V.; Neilan, T.G. HIV Infection and Heart Failure Outcomes in Women. J. Am. Coll. Cardiol. 2017, 69, 107–108. [Google Scholar] [CrossRef]
- Mak, I.T.; Chmielinska, J.J.; Spurney, C.F.; Weglicki, W.B.; Kramer, J.H. Combination ART-Induced Oxidative/Nitrosative Stress, Neurogenic Inflammation and Cardiac Dysfunction in HIV-1 Transgenic (Tg) Rats: Protection by Mg. Int. J. Mol. Sci. 2018, 19, 2409. [Google Scholar] [CrossRef] [PubMed]
- Cheung, J.Y.; Gordon, J.; Wang, J.; Song, J.; Zhang, X.Q.; Tilley, D.G.; Gao, E.; Koch, W.J.; Rabinowitz, J.; Klotman, P.E.; et al. Cardiac Dysfunction in HIV-1 Transgenic Mouse: Role of Stress and BAG3. Clin. Transl. Sci. 2015, 8, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Kelly, K.M.; Tarwater, P.M.; Karper, J.M.; Bedja, D.; Queen, S.E.; Tunin, R.S.; Adams, R.J.; Kass, D.A.; Mankowski, J.L. Diastolic dysfunction is associated with myocardial viral load in simian immunodeficiency virus-infected macaques. AIDS 2012, 26, 815–823. [Google Scholar] [CrossRef]
- Kelly, K.M.; Tocchetti, C.G.; Lyashkov, A.; Tarwater, P.M.; Bedja, D.; Graham, D.R.; Beck, S.E.; Metcalf Pate, K.A.; Queen, S.E.; Adams, R.J.; et al. CCR5 inhibition prevents cardiac dysfunction in the SIV/macaque model of HIV. J. Am. Heart Assoc. 2014, 3, e000874. [Google Scholar] [CrossRef]
- Wu, K.C.; Woldu, B.; Post, W.S.; Hays, A.G. Prevention of heart failure, tachyarrhythmias and sudden cardiac death in HIV. Curr. Opin. HIV AIDS 2022, 17, 261–269. [Google Scholar] [CrossRef]
- Robinson, J.A.; Mahmud, F.J.; Greif, E.; Toribio, M.; Zanni, M.V.; Brown, A.M.; Burdo, T.H. Osteopontin Is an Integral Mediator of Cardiac Interstitial Fibrosis in Models of Human Immunodeficiency Virus Infection. J. Infect. Dis. 2023, 228, 122–132. [Google Scholar] [CrossRef]
- Dash, P.K.; Alomar, F.A.; Hackfort, B.T.; Su, H.; Conaway, A.; Poluektova, L.Y.; Gendelman, H.E.; Gorantla, S.; Bidasee, K.R. HIV-1-Associated Left Ventricular Cardiac Dysfunction in Humanized Mice. Sci. Rep. 2020, 10, 9746. [Google Scholar] [CrossRef]
- What to Start: Initial Combination Antiretroviral Regimens for People with HIV. 2024. Available online: https://clinicalinfo.hiv.gov/en (accessed on 8 April 2025).
- Venter, W.D.F.; Bosch, B.; Sokhela, S.; Akpomiemie, G.; Chandiwana, N.; Tembo, A.; Qavi, A.; Simmons, B.; McCann, K.; Hill, A. Final week 192 results from the ADVANCE trial: First-line TAF/FTC/DTG, TDF/FTC/DTG vs. TDF/FTC/EFV. AIDS 2022. [Google Scholar]
- Bidasee, K.R.; Dash, P.K.; Venn, Z.L.; Zhang, L.; Bidasee, S.R.; Tu, R.; Hackfort, B.T.; Guo, L.; Gorantla, S. Diastolic Dysfunction With Preserved Ejection Fraction in Humanized HIV-Female Mice on cART. In Proceedings of the Conference on Retroviruses and Opportunistic Infections (CROI), Denver, CO, Canada, 3–6 March 2024. [Google Scholar]
- Theron, A.J.; Anderson, R.; Madzime, M.; Rossouw, T.M.; Steel, H.C.; Meyer, P.W.A.; Cholo, M.C.; Kwofie, L.L.I.; Feldman, C.; Tintinger, G.R. Pro-Inflammatory Interactions of Dolutegravir with Human Neutrophils in an In Vitro Study. Molecules 2022, 27, 9057. [Google Scholar] [CrossRef]
- Maandi, S.C.; Maandi, M.T.; Patel, A.; Manville, R.W.; Mabley, J.G. Divergent effects of HIV reverse transcriptase inhibitors on pancreatic beta-cell function and survival: Potential role of oxidative stress and mitochondrial dysfunction. Life Sci. 2022, 294, 120329. [Google Scholar] [CrossRef] [PubMed]
- Kierans, S.J.; Taylor, C.T. Regulation of glycolysis by the hypoxia-inducible factor (HIF): Implications for cellular physiology. J. Physiol. 2021, 599, 23–37. [Google Scholar] [CrossRef]
- Stone, L.; Looby, S.E.; Zanni, M.V. Cardiovascular disease risk among women living with HIV in North America and Europe. Curr. Opin. HIV AIDS 2017, 12, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Kentoffio, K.; Temu, T.M.; Shakil, S.S.; Zanni, M.V.; Longenecker, C.T. Cardiovascular disease risk in women living with HIV. Curr. Opin. HIV AIDS 2022, 17, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Mutengo, K.H.; Lima, B.B.; Mutale, W.; Mweemba, A.; Kabwe, L.; Banda, C.; Kaayunga, C.; Mulenga, M.; Heimburger, D.; Masenga, S.K.; et al. The influence of HIV infection on myocardial fibrosis diagnosed by cardiac magnetic resonance imaging in adults: A systematic review and meta-analysis of observation studies. Front. Cardiovasc. Med. 2025, 12, 1534533. [Google Scholar] [CrossRef]
- Ntsekhe, M.; Baker, J.V. Cardiovascular Disease Among Persons Living With HIV: New Insights Into Pathogenesis and Clinical Manifestations in a Global Context. Circulation 2023, 147, 83–100. [Google Scholar] [CrossRef]
- Abelman, R.A.; Mugo, B.M.; Zanni, M.V. Conceptualizing the Risks of Coronary Heart Disease and Heart Failure Among People Aging with HIV: Sex-Specific Considerations. Curr. Treat. Options Cardiovasc. Med. 2019, 21, 41. [Google Scholar] [CrossRef]
- Little, W.C.; Oh, J.K. Echocardiographic evaluation of diastolic function can be used to guide clinical care. Circulation 2009, 120, 802–809. [Google Scholar] [CrossRef]
- Robinson, S.; Ring, L.; Oxborough, D.; Harkness, A.; Bennett, S.; Rana, B.; Sutaria, N.; Lo Giudice, F.; Shun-Shin, M.; Paton, M.; et al. The assessment of left ventricular diastolic function: Guidance and recommendations from the British Society of Echocardiography. Echo Res. Pract. 2024, 11, 16. [Google Scholar] [CrossRef]
- Tseng, A.S.; Gorsi, U.S.; Barros-Gomes, S.; Miller, F.A.; Pellikka, P.A.; Clavell, A.L.; Villarraga, H.R. Use of speckle-tracking echocardiography-derived strain and systolic strain rate measurements to predict rejection in transplant hearts with preserved ejection fraction. BMC Cardiovasc. Disord. 2018, 18, 241. [Google Scholar] [CrossRef]
- Sasson, Z.; Rasooly, Y.; Gupta, R.; Rasooly, I. Left atrial enlargement in healthy obese: Prevalence and relation to left ventricular mass and diastolic function. Can. J. Cardiol. 1996, 12, 257–263. [Google Scholar] [PubMed]
- Pawar, S.G.; Saravanan, P.B.; Gulati, S.; Pati, S.; Joshi, M.; Salam, A.; Khan, N. Study the relationship between left atrial (LA) volume and left ventricular (LV) diastolic dysfunction and LV hypertrophy: Correlate LA volume with cardiovascular risk factors. Dis. Mon. 2024, 70, 101675. [Google Scholar] [CrossRef]
- Reimer, K.A.; Jennings, R.B.; Tatum, A.H. Pathobiology of acute myocardial ischemia: Metabolic, functional and ultrastructural studies. Am. J. Cardiol. 1983, 52, 72A–81A. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, N.; Yamamoto, K.; Abe, T.; Yasuoka, N.; Takamune, N.; Misumi, S. Glucose-dependent aerobic glycolysis contributes to recruiting viral components into HIV-1 particles to maintain infectivity. Biochem. Biophys. Res. Commun. 2021, 549, 187–193. [Google Scholar] [CrossRef]
- Zhang, X.; Zink, F.; Hezel, F.; Vogt, J.; Wachter, U.; Wepler, M.; Loconte, M.; Kranz, C.; Hellmann, A.; Mizaikoff, B.; et al. Metabolic substrate utilization in stress-induced immune cells. Intensive Care Med. Exp. 2020, 8 (Suppl. S1), 28. [Google Scholar] [CrossRef] [PubMed]
- Frangogiannis, N.G. Pathophysiology of Myocardial Infarction. Compr. Physiol. 2015, 5, 1841–1875. [Google Scholar] [CrossRef]
- Donahue, J.K.; Chrispin, J.; Ajijola, O.A. Mechanism of Ventricular Tachycardia Occurring in Chronic Myocardial Infarction Scar. Circ. Res. 2024, 134, 328–342. [Google Scholar] [CrossRef]
- Dagur, R.S.; Wang, W.; Cheng, Y.; Makarov, E.; Ganesan, M.; Suemizu, H.; Gebhart, C.L.; Gorantla, S.; Osna, N.; Poluektova, L.Y. Human hepatocyte depletion in the presence of HIV-1 infection in dual reconstituted humanized mice. Biol. Open 2018, 7, bio029785. [Google Scholar] [CrossRef]
- Li, Y.Y.; Feldman, A.M. Matrix metalloproteinases in the progression of heart failure: Potential therapeutic implications. Drugs 2001, 61, 1239–1252. [Google Scholar] [CrossRef]
- Kim, J.; Kim, O.S.; Kim, C.S.; Kim, N.H.; Kim, J.S. Cytotoxic role of methylglyoxal in rat retinal pericytes: Involvement of a nuclear factor-kappaB and inducible nitric oxide synthase pathway. Chem. Biol. Interact. 2010, 188, 86–93. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, J.; Zheng, Y.; Jiang, J.; Wang, L.; Wu, J.; Zhang, C.; Luo, M. Glucose metabolite methylglyoxal induces vascular endothelial cell pyroptosis via NLRP3 inflammasome activation and oxidative stress in vitro and in vivo. Cell. Mol. Life Sci. 2024, 81, 401. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, A.; Samtani, R.; Dhanantwari, P.; Lee, E.; Yamada, S.; Shiota, K.; Donofrio, M.T.; Leatherbury, L.; Lo, C.W. A detailed comparison of mouse and human cardiac development. Pediatr. Res. 2014, 76, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Weichseldorfer, M.; Heredia, A.; Reitz, M.; Bryant, J.L.; Latinovic, O.S. Use of Humanized Mouse Models for Studying HIV-1 Infection, Pathogenesis and Persistence. J. AIDS HIV Treat. 2020, 2, 23–29. [Google Scholar] [PubMed]
- Gorantla, S.; Makarov, E.; Finke-Dwyer, J.; Castanedo, A.; Holguin, A.; Gebhart, C.L.; Gendelman, H.E.; Poluektova, L. Links between progressive HIV-1 infection of humanized mice and viral neuropathogenesis. Am. J. Pathol. 2010, 177, 2938–2949. [Google Scholar] [CrossRef]

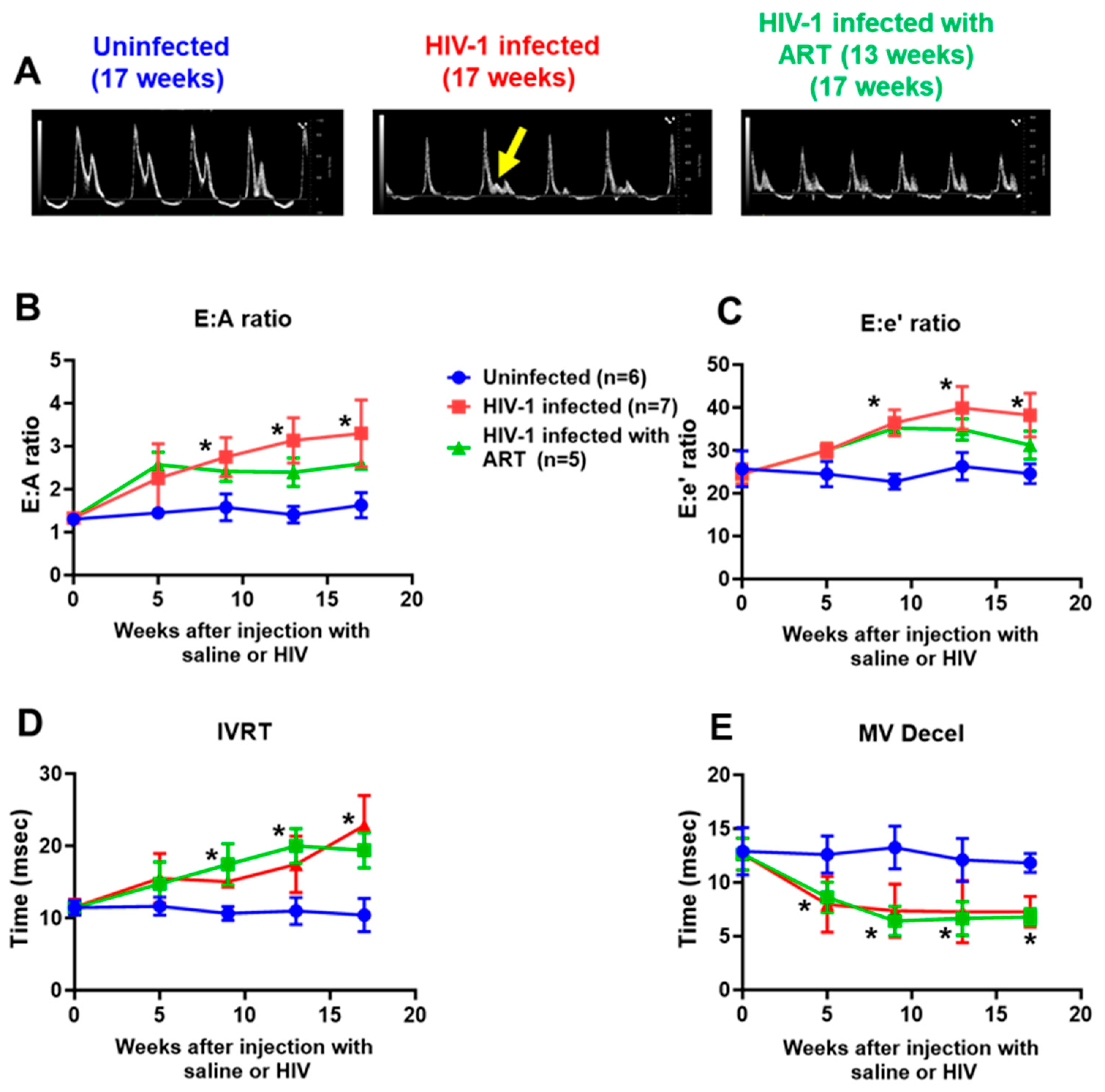

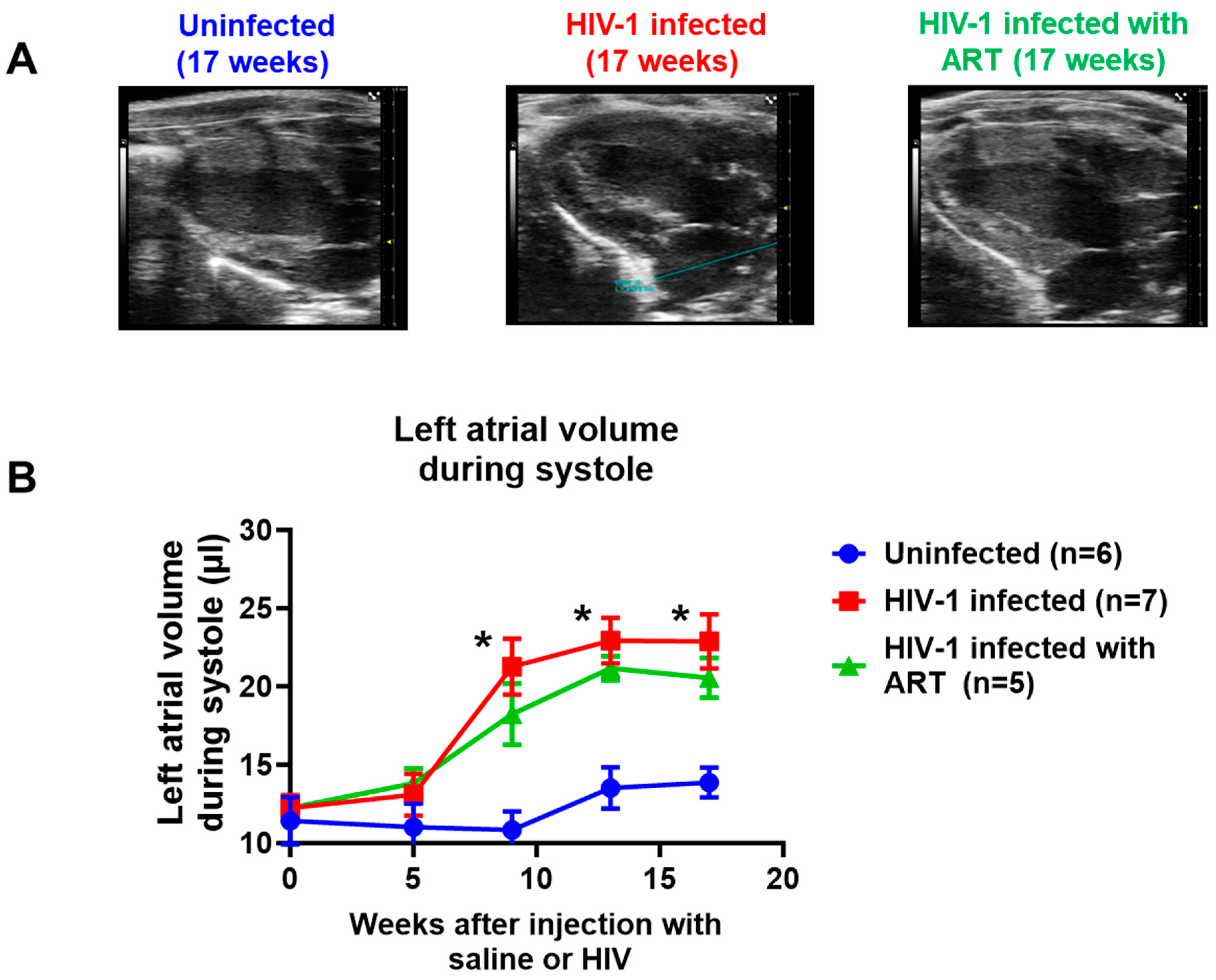
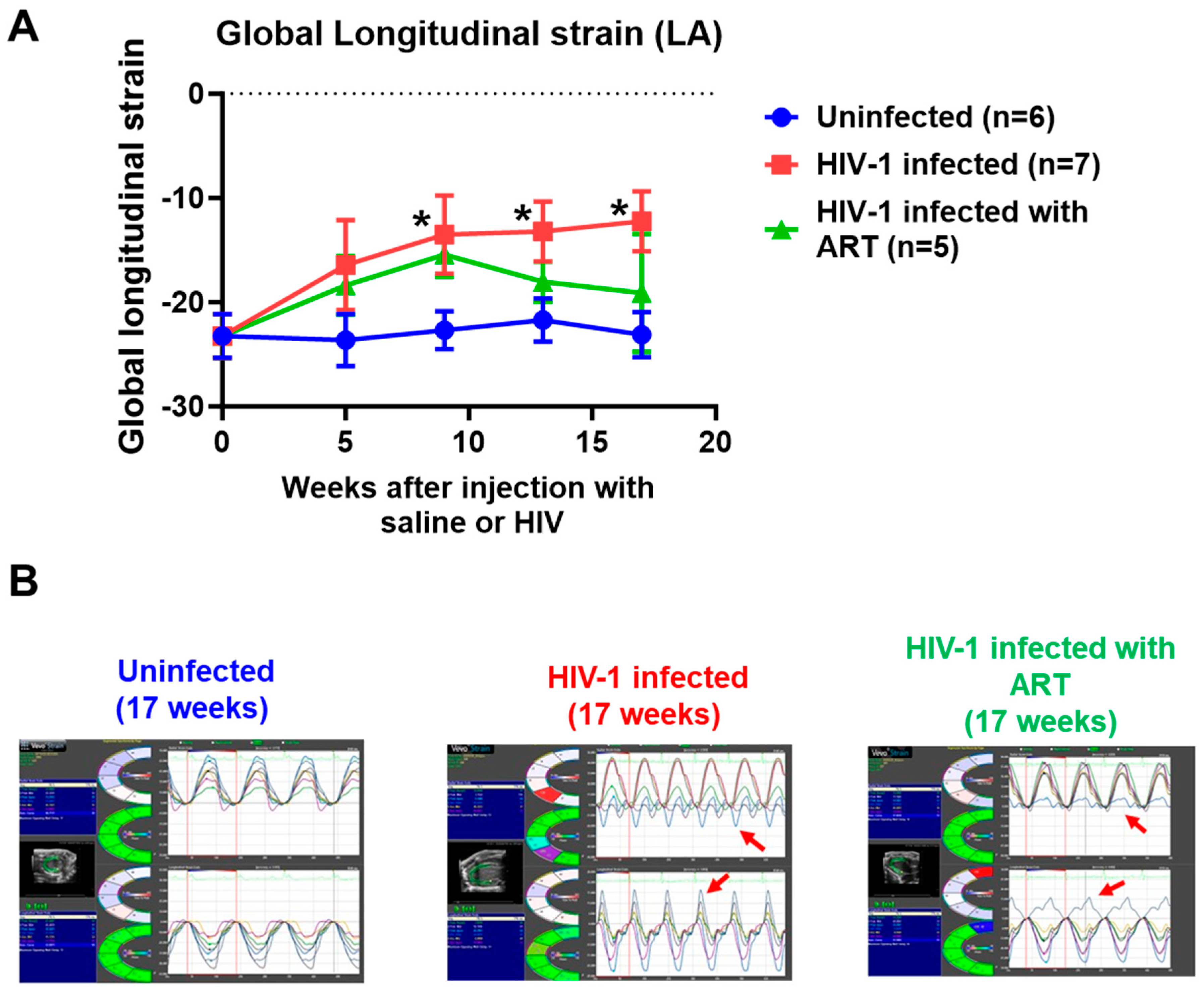
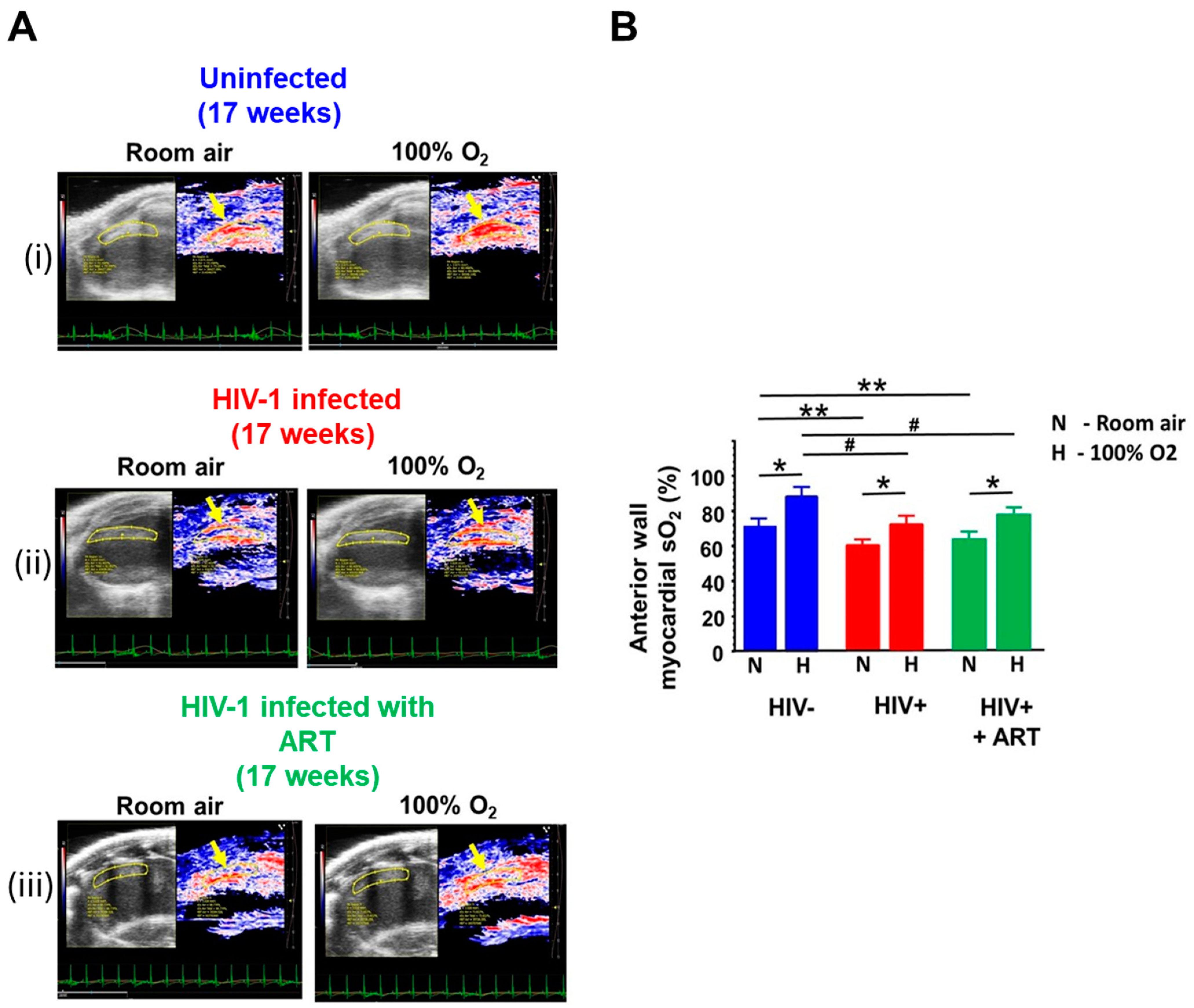


| Parameters | At Start (t = 0 weeks) | End (t = 17 weeks) | |
|---|---|---|---|
| Hu-NSG mice (n = 6) | Body weight (g) | 18.3 ± 0.4 | 19.2 ± 1.0 |
| Plasma HSA-MG (μg/mL) | 26.6 ± 3.4 | 34.1 ± 4.4 | |
| Plasma SSAO activity (units/mL/2 h) | 3.1 ± 0.3 | 3.2 ± 0.4 | |
| Heart weight (mg) | NA | 156.2 ± 3.2 | |
| Hu-NSG mice infected with HIV-1ADA (n = 6) | Body weight (g) | 18.3 ± 0.3 | 18.9 ± 1.0 |
| Plasma HIV viral load (RNA copies/mL) | 1.0 × 105 (injected) | 1.6 ± 0.2 × 106 | |
| Plasma HSA-MG (μg/mL) | 25.2 ± 3.2 | 59.6 ± 5.4 * | |
| Plasma SSAO activity (units/mL/2 h) | 3.1 ± 0.3 | 6.2 ± 0.2 * | |
| Heart weight (mg) | NA | 143.4 ± 4.2 * | |
| Hu-NSG mice Infected with HIV-1ADA and treated with DTG/TDF/FTC | Body weight (g) | 18.3 ± 0.3 | 19.1 ± 1.0 |
| Plasma HIV viral load (RNA copies/mL) | 1.0 × 105 (injected) | 0.5 ± 0.1 × 102 | |
| Plasma HSA-MG (μg/mL) | 27.2 ± 2.2 | 58.1 ± 8.4 * | |
| Plasma SSAO activity (units/mL/2 h) | 3.2 ± 0.1 | 6.1 ± 0.2 * | |
| Heart weight (mg) | NA | 146.5 ± 4.2 * | |
| Plasma DTG (ng/mL) | NA | 7622.5 ± 665.6 | |
| Plasma TDF (ng/mL) | NA | 546.7 ± 74.5 | |
| Plasma FTC (ng/mL) | NA | 1818.4 ± 278.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alomar, F.A.; Dash, P.K.; Ramasamy, M.; Venn, Z.L.; Bidasee, S.R.; Zhang, C.; Hackfort, B.T.; Gorantla, S.; Bidasee, K.R. Diastolic Dysfunction with Vascular Deficits in HIV-1-Infected Female Humanized Mice Treated with Antiretroviral Drugs. Int. J. Mol. Sci. 2025, 26, 3801. https://doi.org/10.3390/ijms26083801
Alomar FA, Dash PK, Ramasamy M, Venn ZL, Bidasee SR, Zhang C, Hackfort BT, Gorantla S, Bidasee KR. Diastolic Dysfunction with Vascular Deficits in HIV-1-Infected Female Humanized Mice Treated with Antiretroviral Drugs. International Journal of Molecular Sciences. 2025; 26(8):3801. https://doi.org/10.3390/ijms26083801
Chicago/Turabian StyleAlomar, Fadhel A., Prasanta K. Dash, Mahendran Ramasamy, Zachary L. Venn, Sean R. Bidasee, Chen Zhang, Bryan T. Hackfort, Santhi Gorantla, and Keshore R. Bidasee. 2025. "Diastolic Dysfunction with Vascular Deficits in HIV-1-Infected Female Humanized Mice Treated with Antiretroviral Drugs" International Journal of Molecular Sciences 26, no. 8: 3801. https://doi.org/10.3390/ijms26083801
APA StyleAlomar, F. A., Dash, P. K., Ramasamy, M., Venn, Z. L., Bidasee, S. R., Zhang, C., Hackfort, B. T., Gorantla, S., & Bidasee, K. R. (2025). Diastolic Dysfunction with Vascular Deficits in HIV-1-Infected Female Humanized Mice Treated with Antiretroviral Drugs. International Journal of Molecular Sciences, 26(8), 3801. https://doi.org/10.3390/ijms26083801









