Co-Encapsulation of Phycocyanin and Albumin-Bound Curcumin in Biopolymeric Hydrogels
Abstract
:1. Introduction
2. Results
2.1. Phycocyanin Extraction and Purification
2.2. Hydrogel Characterization
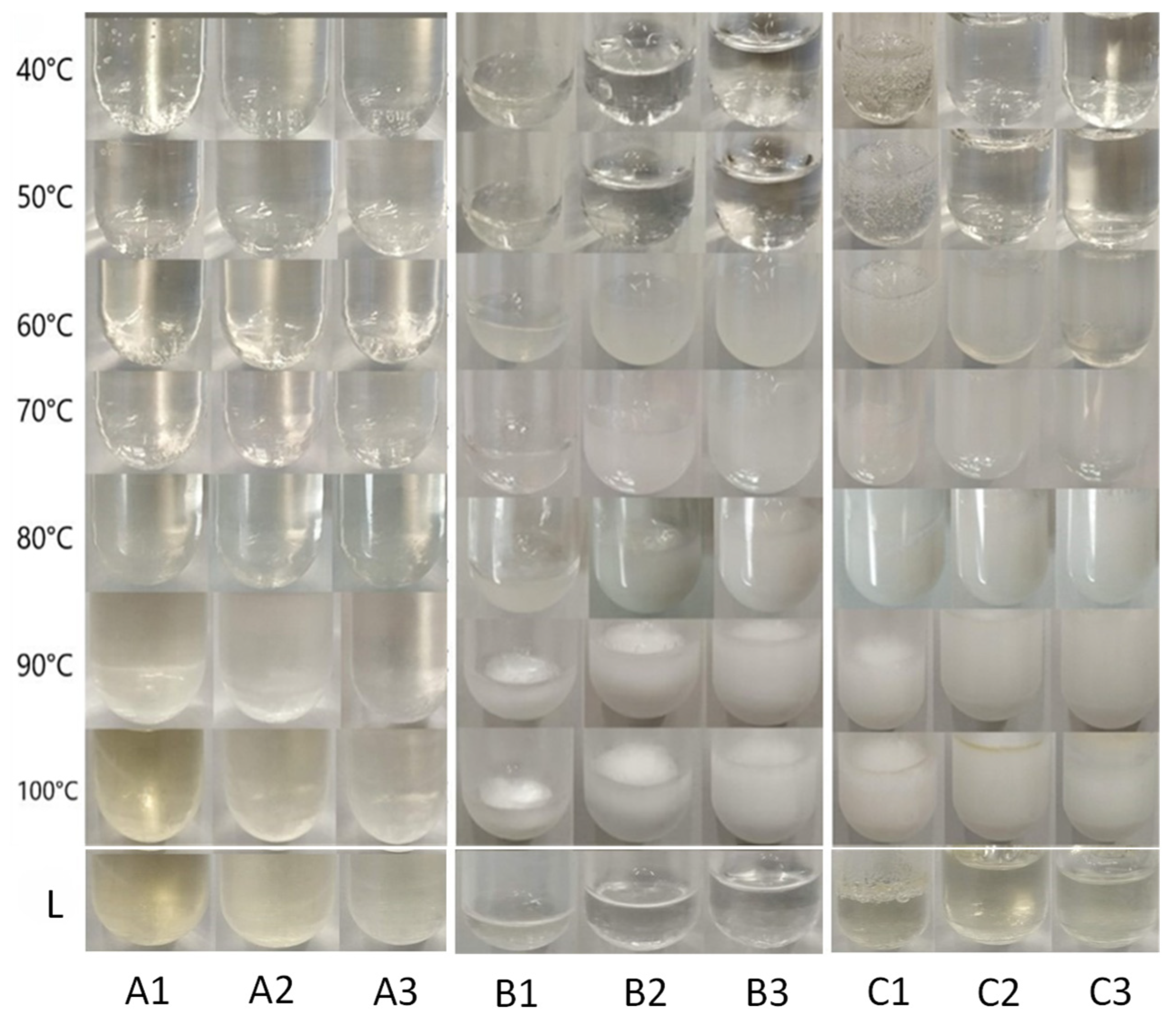
2.2.1. Heating Treatment
Chitosan Hydrogels
HPMC Hydrogels
Chitosan–HPMC Hydrogels
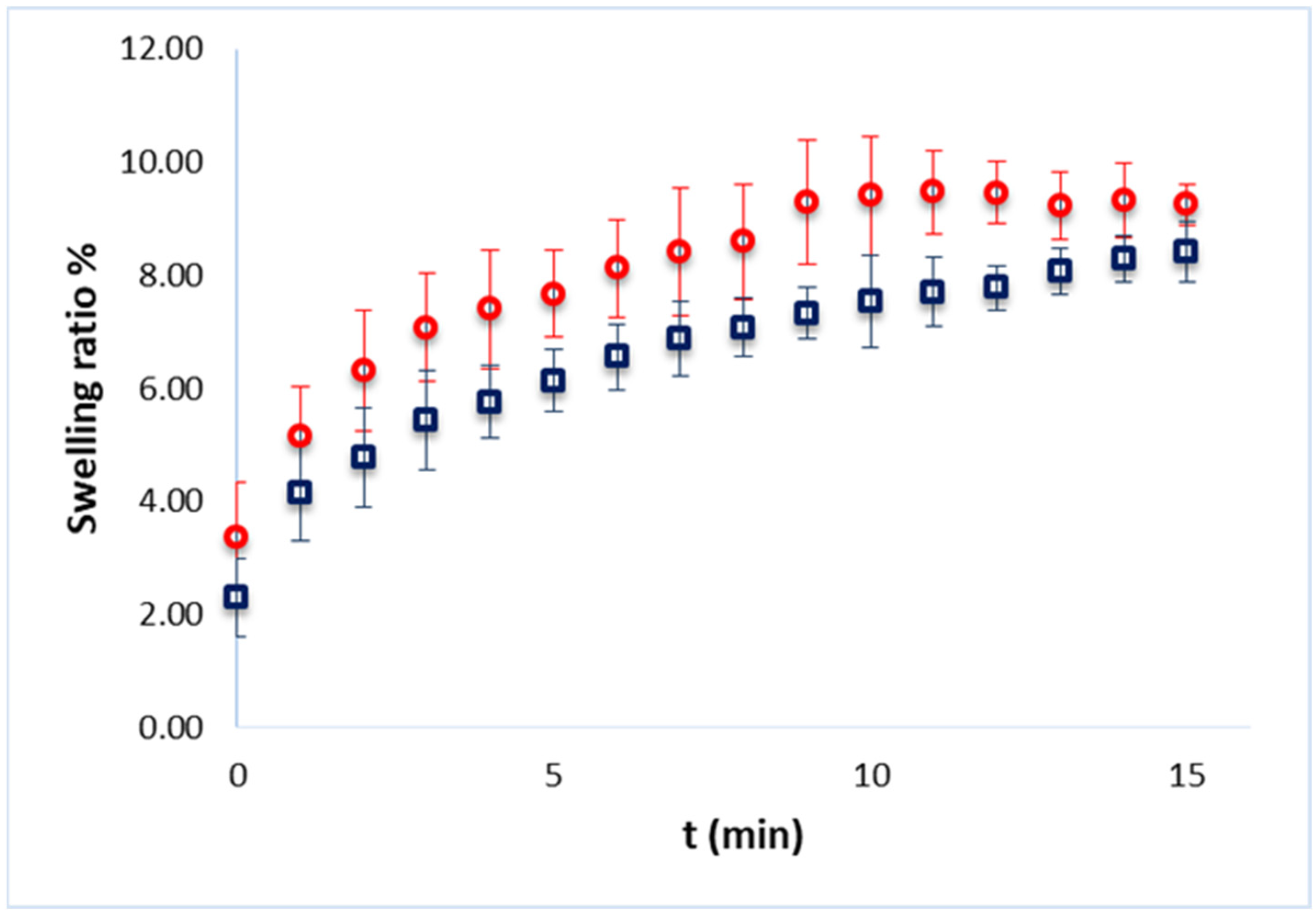
2.2.2. Swelling Ratio
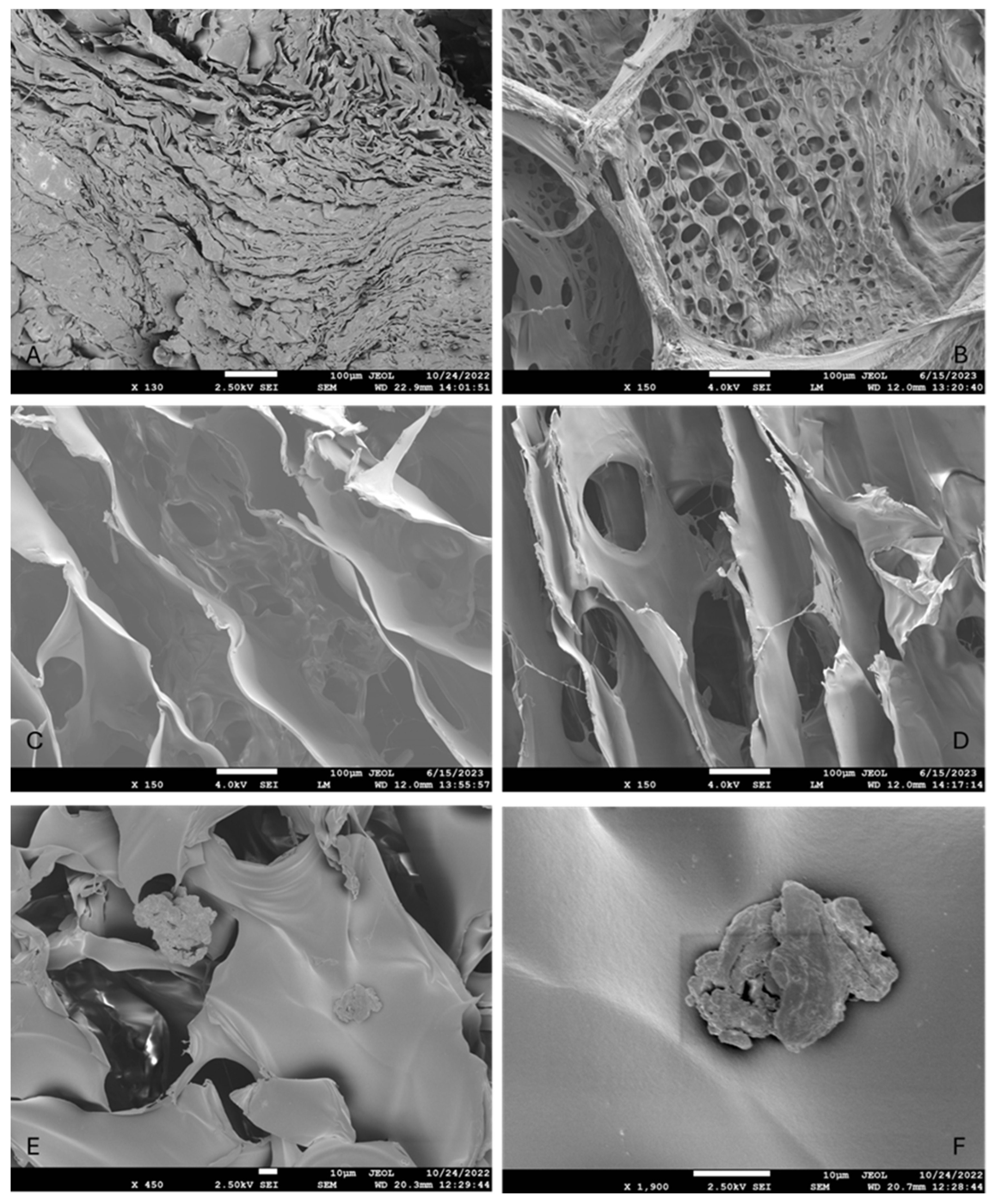
2.2.3. Morphological Analysis
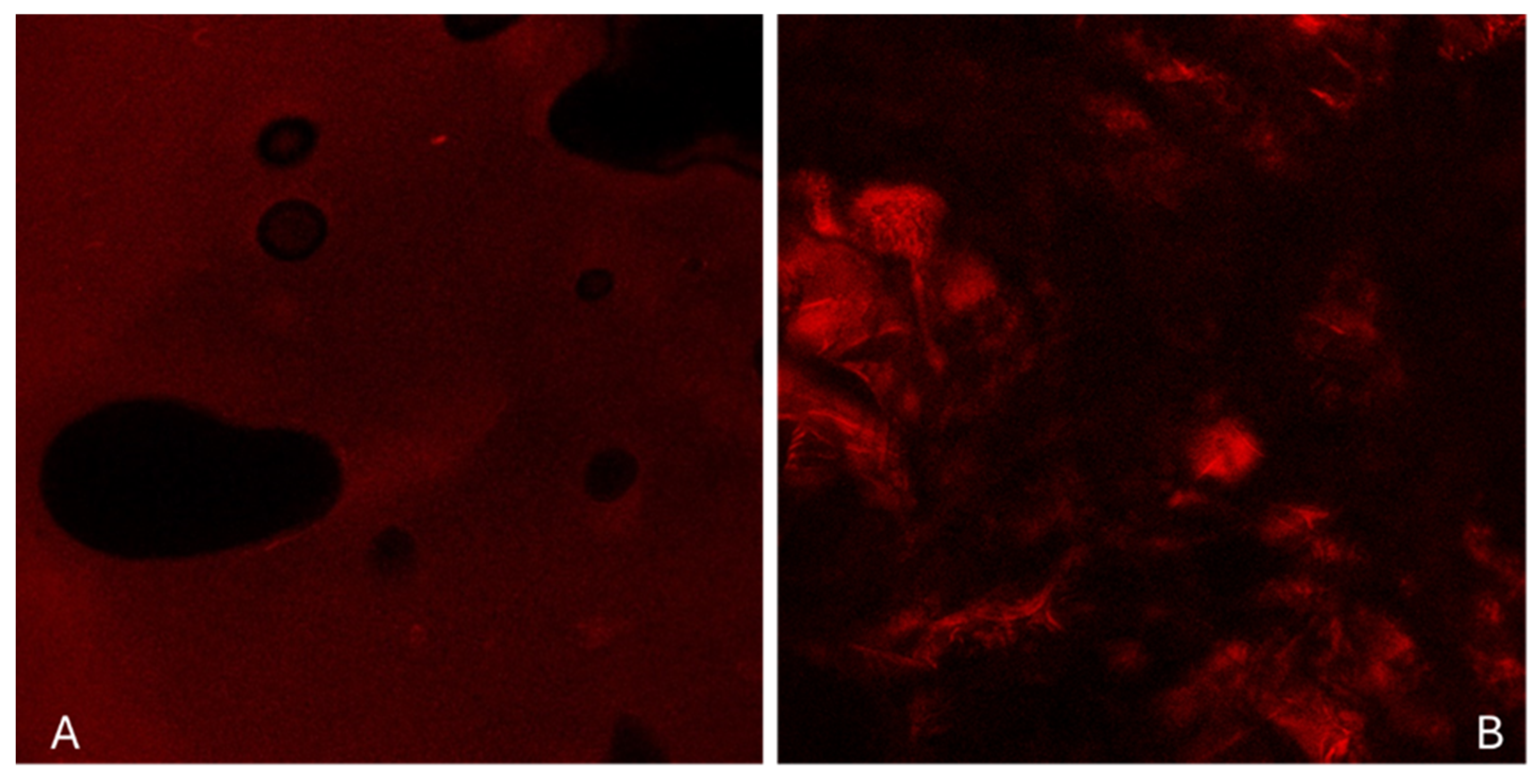
2.2.4. Structural Characterization
2.3. Antioxidant Ability Study
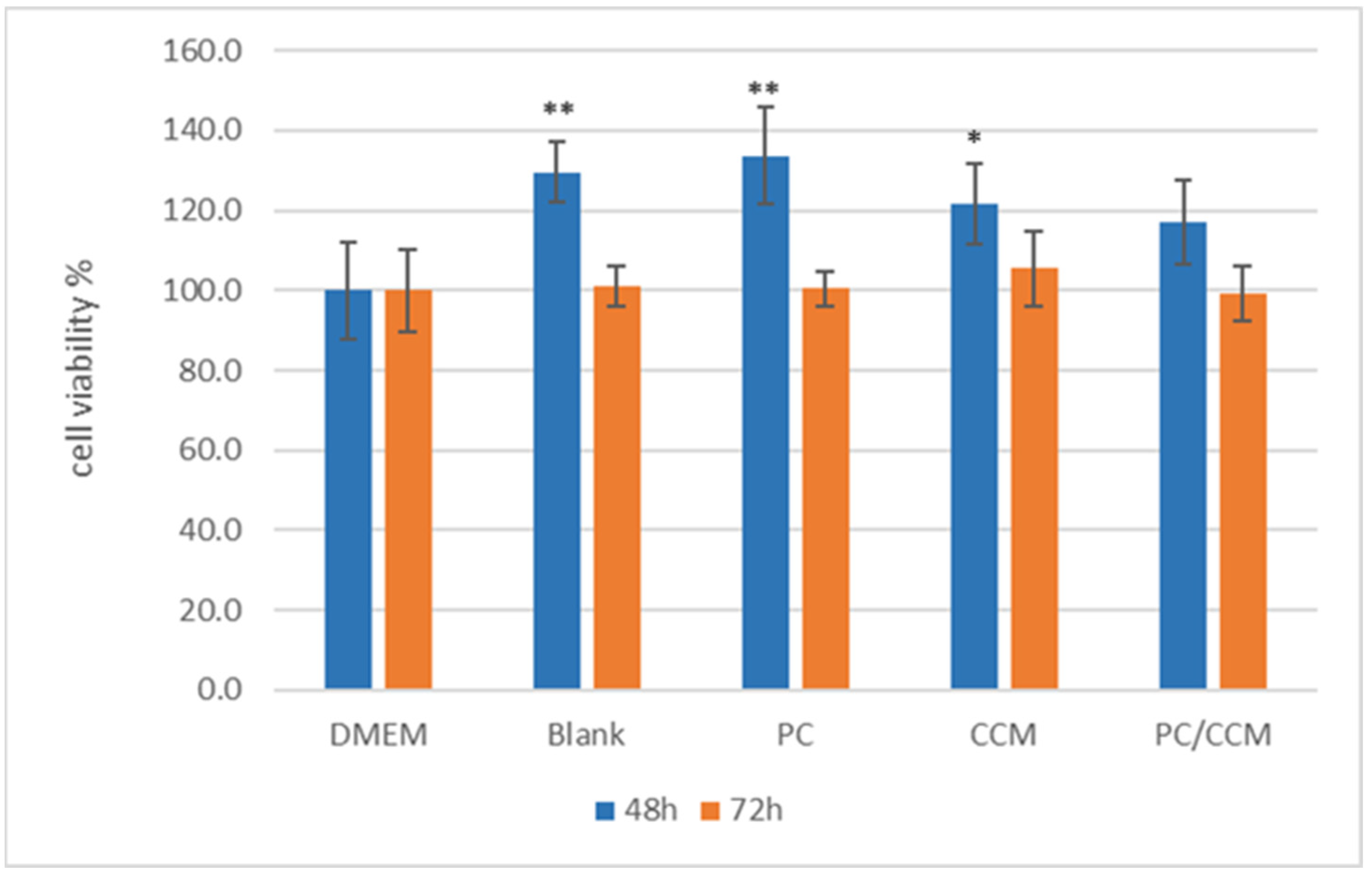
2.4. Cell Viability Assay
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Phycocyanin Extraction and Purification
4.2.2. CCM/BSA Protein Carrier Formulation
4.2.3. Hydrogel Formulation and Encapsulation of Antioxidants
Hydrogel Preparation
Encapsulation of Antioxidants in Hydrogels
4.2.4. Hydrogel Structural Characterization
Thermal Stability
Swelling Ratio
Scanning Electron Microscopy (SEM)
Structural Characterization
4.2.5. Antioxidant Assessment by DPPH Colorimetric Assay
4.2.6. Cell Culture and Cell Proliferation Assay
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HPMC | (Hydroxypropyl)methyl cellulose |
| PC | Phycocyanin |
| CCM | Curcumin |
| BSA | Bovine serum albumin |
| AS | Ammonium sulfate |
| PB | Phosphate buffer |
| DPPH | 2,2-diphenyl-1-picryl-hydrazyl-hydrate |
| SEM | Scanning electron microscopy |
| CLSM | Confocal scanning electron microscopy |
| DMEM | Dulbecco’s Modified Eagle’s Medium |
| MTT | 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide |
References
- Kaith, B.S.; Singh, A.; Sharma, A.K.; Sud, D. Hydrogels: Synthesis, classification, properties and potential applications—A brief review. J. Polym. Environ. 2021, 29, 3827–3841. [Google Scholar] [CrossRef]
- Maleki, B.; Kargar, P.G.; Ashrafi, S.S.; Ghani, M. Perspective Chapter: Introduction to Hydrogels–Definition, Classifications, Applications and Methods of Preparation; IntechOpen: London, UK, 2024. [Google Scholar]
- Fu, J.; Yang, F.; Guo, Z. The chitosan hydrogels: From structure to function. New J. Chem. 2018, 42, 17162–17180. [Google Scholar] [CrossRef]
- Bashir, S.; Zafar, N.; Lebaz, N.; Mahmood, A.; Elaissari, A. Hydroxypropyl methylcellulose-based hydrogel copolymeric for controlled delivery of galantamine hydrobromide in Dementia. Processes 2020, 8, 1350. [Google Scholar] [CrossRef]
- Chen, C.-P.; Hsieh, C.-M.; Tsai, T.; Yang, J.-C.; Chen, C.-T. Optimization and evaluation of a chitosan/hydroxypropyl methylcellulose hydrogel containing toluidine blue O for antimicrobial photodynamic inactivation. Int. J. Mol. Sci. 2015, 16, 20859–20872. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, H.; Jiang, L.; Tao, Y.; Song, Y.; Yu, S.; Wang, T.; Feng, N. Preparation of cellulose hydrogel dressing with evenly dispersed hydrophobic drugs by hydrogen bonding and encapsulation methods. Macromol. Mater. Eng. 2021, 306, 2100286. [Google Scholar] [CrossRef]
- Huang, B.; Lu, M.; Wang, D.; Song, Y.; Zhou, L. Versatile magnetic gel from peach gum polysaccharide for efficient adsorption of Pb2+ and Cd2+ ions and catalysis. Carbohydr. Polym. 2018, 181, 785–792. [Google Scholar] [CrossRef]
- Su, C.-W.; Chiang, C.-S.; Li, W.-M.; Hu, S.-H.; Chen, S.-Y. Multifunctional nanocarriers for simultaneous encapsulation of hydrophobic and hydrophilic drugs in cancer treatment. Nanomedicine 2014, 9, 1499–1515. [Google Scholar] [CrossRef]
- Demisli, S.; Galani, E.; Goulielmaki, M.; Kyrilis, F.L.; Ilić, T.; Hamdi, F.; Crevar, M.; Kastritis, P.L.; Pletsa, V.; Nallet, F. Encapsulation of cannabidiol in oil-in-water nanoemulsions and nanoemulsion-filled hydrogels: A structure and biological assessment study. J. Colloid Interface Sci. 2023, 634, 300–313. [Google Scholar] [CrossRef]
- Alshangiti, D.M.; El-Damhougy, T.K.; Zaher, A.; Madani, M. Revolutionizing biomedicine: Advancements, applications, and prospects of nanocomposite macromolecular carbohydrate-based hydrogel biomaterials: A review. RSC Adv. 2023, 13, 35251–35291. [Google Scholar] [CrossRef]
- Matskou, K.; Kisaoglan, B.; Mavroidi, B.; Pelecanou, M.; Zoumpanioti, M.; Matis, I.; Xenakis, A. Inducing the formation of a colloidal albumin carrier of curcumin. JCIS Open 2022, 6, 100051. [Google Scholar] [CrossRef]
- Kim, T.H.; Jiang, H.H.; Youn, Y.S.; Park, C.W.; Tak, K.K.; Lee, S.; Kim, H.; Jon, S.; Chen, X.; Lee, K.C. Preparation and characterization of water-soluble albumin-bound curcumin nanoparticles with improved antitumor activity. Int. J. Pharm. 2011, 403, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Manirafasha, E.; Guo, L.; Jing, K. Nutraceutical and pharmaceutical applications of phycobiliproteins. In Pigments from Microalgae Handbook; Springer: Cham, Switzerland, 2020; pp. 575–584. [Google Scholar]
- Sotiroudis, T.G.; Sotiroudis, G.T. Health aspects of Spirulina (Arthrospira) microalga food supplement. J. Serbian Chem. Soc. 2013, 78, 395–405. [Google Scholar] [CrossRef]
- Kuhnholz, J.; Glockow, T.; Siebecke, V.; Le, A.T.; Tran, L.-D.; Noke, A. Comparison of different methods for extraction of phycocyanin from the cyanobacterium Arthrospira maxima (Spirulina). J. Appl. Phycol. 2024, 36, 1725–1735. [Google Scholar] [CrossRef]
- Khandual, S.; Sanchez, E.O.L.; Andrews, H.E.; De la Rosa, J.D.P. Phycocyanin content and nutritional profile of Arthrospira platensis from Mexico: Efficient extraction process and stability evaluation of phycocyanin. BMC Chem. 2021, 15, 24. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Lin, Z.; Yang, Y.; Jiang, T.; Shang, J.; Luo, Z. Biocompatible conductive hydrogels: Applications in the field of biomedicine. Int. J. Mol. Sci. 2022, 23, 4578. [Google Scholar] [CrossRef]
- Georgieva, V.; Zvezdova, D.; Vlaev, L. Non-isothermal kinetics of thermal degradation of chitosan. Chem. Cent. J. 2012, 6, 81. [Google Scholar] [CrossRef]
- Villar-Chavero, M.M.; Domínguez, J.C.; Alonso, M.V.; Oliet, M.; Rodriguez, F. Thermal and kinetics of the degradation of chitosan with different deacetylation degrees under oxidizing atmosphere. Thermochim. Acta 2018, 670, 18–26. [Google Scholar] [CrossRef]
- Silva, S.M.; Pinto, F.V.; Antunes, F.E.; Miguel, M.G.; Sousa, J.J.; Pais, A.A. Aggregation and gelation in hydroxypropylmethyl cellulose aqueous solutions. J. Colloid Interface Sci. 2008, 327, 333–340. [Google Scholar] [CrossRef]
- Ramli, N.A.; Adam, F.; Mohd Amin, K.N.; Nor, A.M.; Ries, M.E. Evaluation of mechanical and thermal properties of carrageenan/hydroxypropyl methyl cellulose hard capsule. Can. J. Chem. Eng. 2023, 101, 1219–1234. [Google Scholar] [CrossRef]
- Zaccarona, C.; Guiotoku, M.; Oliveira, R. Thermal degradation of hydroxypropylmethylcellulose and sodium alginate blends under an inert atmosphere. In Proceedings of the 5th International Symposium on Natural Polymers, São Pedro, Brazil, 2004; pp. 4–6. Available online: https://www.eucalyptus.com.br/artigos/2004_Eigth+Symposium+Lignins+Wood+Components_Arquivo+18.pdf (accessed on 16 January 2025).
- Vassiliadi, E.; Tsirigotis-Maniecka, M.; Symons, H.E.; Gobbo, P.; Nallet, F.; Xenakis, A.; Zoumpanioti, M. (Hydroxypropyl) methyl Cellulose-Chitosan Film as a Matrix for Lipase Immobilization—Part ΙΙ: Structural Studies. Gels 2022, 8, 595. [Google Scholar] [CrossRef]
- Vassiliadi, E.; Mitsou, E.; Avramiotis, S.; Chochos, C.L.; Pirolt, F.; Medebach, M.; Glatter, O.; Xenakis, A.; Zoumpanioti, M. Structural study of (Hydroxypropyl) methyl cellulose microemulsion-based gels used for biocompatible encapsulations. Nanomaterials 2020, 10, 2204. [Google Scholar] [CrossRef]
- Dandavate, V.; Madamwar, D. Reusability of surfactant-coated Candida rugosa lipase immobilized in gelatin microemulsion-based organogels for ethyl isovalerate synthesis. J. Microbiol. Biotechnol. 2008, 18, 735–741. [Google Scholar] [PubMed]
- Meng, D.; Zhang, L.; Wang, Q.; Zhang, Y.; Sun, Y.; Zhang, H.; Wang, Z.; Zhou, Z.; Yang, R. Self-assembly of phycoerythrin with oligochitosan by electrostatic interaction for stabilization of phycoerythrin. J. Agric. Food Chem. 2021, 69, 12818–12827. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Chen, L.; Shen, T.; Wu, D. Preparation and properties of a novel thermo-sensitive hydrogel based on chitosan/hydroxypropyl methylcellulose/glycerol. Int. J. Biol. Macromol. 2016, 93, 775–782. [Google Scholar] [CrossRef]
- Li, H.; Zhao, L.; Chen, X.D.; Mercadé-Prieto, R. Swelling of whey and egg white protein hydrogels with stranded and particulate microstructures. Int. J. Biol. Macromol. 2016, 83, 152–159. [Google Scholar]
- Ivanova, D.G.; Yaneva, Z.L. Antioxidant properties and redox-modulating activity of chitosan and its derivatives: Biomaterials with application in cancer therapy. BioResearch Open Access 2020, 9, 64–72. [Google Scholar] [PubMed]
- Grover, P.; Bhatnagar, A.; Kumari, N.; Bhatt, A.N.; Nishad, D.K.; Purkayastha, J. C-Phycocyanin-a novel protein from Spirulina platensis-In vivo toxicity, antioxidant and immunomodulatory studies. Saudi J. Biol. Sci. 2021, 28, 1853–1859. [Google Scholar] [CrossRef]
- Muthu, M.; Gopal, J.; Chun, S.; Devadoss, A.J.P.; Hasan, N.; Sivanesan, I. Crustacean waste-derived chitosan: Antioxidant properties and future perspective. Antioxidants 2021, 10, 228. [Google Scholar] [CrossRef]
- Lou, J.; Mooney, D.J. Chemical strategies to engineer hydrogels for cell culture. Nat. Rev. Chem. 2022, 6, 726–744. [Google Scholar] [CrossRef]
- Gao, L.; Gan, H.; Meng, Z.; Gu, R.; Wu, Z.; Zhang, L.; Zhu, X.; Sun, W.; Li, J.; Zheng, Y. Effects of genipin cross-linking of chitosan hydrogels on cellular adhesion and viability. Colloids Surf. B Biointerfaces 2014, 117, 398–405. [Google Scholar] [CrossRef]
- Zhong, C.; Wu, J.; Reinhart-King, C.; Chu, C. Synthesis, characterization and cytotoxicity of photo-crosslinked maleic chitosan–polyethylene glycol diacrylate hybrid hydrogels. Acta Biomater. 2010, 6, 3908–3918. [Google Scholar] [CrossRef] [PubMed]
- Tibbitt, M.W.; Anseth, K.S. Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnol. Bioeng. 2009, 103, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Pan-utai, W.; Iamtham, S. Physical extraction and extrusion entrapment of C-phycocyanin from Arthrospira platensis. J. King Saud Univ.-Sci. 2019, 31, 1535–1542. [Google Scholar] [CrossRef]
- Satani, H.; Kuwata, M.; Ishii, H.; Inoue, T.; Shimizu, A. Preparation of SEM hydrogel samples using a high pressure water freeze fracture method. High Press. Res. 2021, 41, 97–108. [Google Scholar] [CrossRef]
- Leney, A.C.; Tschanz, A.; Heck, A.J. Connecting color with assembly in the fluorescent B-phycoerythrin protein complex. FEBS J. 2018, 285, 178–187. [Google Scholar] [CrossRef]
- Theochari, I.; Papadimitriou, V.; Papahatjis, D.; Assimomytis, N.; Pappou, E.; Pratsinis, H.; Xenakis, A.; Pletsa, V. Oil-in-water microemulsions as hosts for benzothiophene-based cytotoxic compounds: An effective combination. Biomimetics 2018, 3, 13. [Google Scholar] [CrossRef]
- Reay, S.L.; Jackson, E.L.; Ferreira, A.M.; Hilkens, C.M.; Novakovic, K. In vitro evaluation of the biodegradability of chitosan–genipin hydrogels. Mater. Adv. 2022, 3, 7946–7959. [Google Scholar] [CrossRef]
| Hydrogel | Polymer Weight % (w/w) | |
|---|---|---|
| Chitosan | HPMC | |
| A1 | 3.2 | - |
| A2 | 2.4 | - |
| A3 | 2.0 | - |
| B1 | - | 16.7 |
| B2 | - | 9.1 |
| B3 | - | 6.3 |
| C1 | 1.2 | 2.1 |
| C2 | 1.0 | 1.0 |
| C3 | 0.8 | 0.8 |
| C4 | 1.7 | 1.7 |
| % Reduction of DPPH | ||
|---|---|---|
| In Solution | In Hydrogel | |
| Empty hydrogel | - | 56 ± 0.01 |
| PC | 44 ± 0.04 | 54 ± 0.07 |
| CCM/BSA | 48 ± 0.07 | 71 ± 0.09 |
| PC and CCM/BSA | 58 ± 0.01 | 70 ± 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matskou, K.; Matis, I.; Demisli, S.; Rigkos, K.; Karandrea, E.; Kourioti, K.; Sotiroudis, G.; Pletsa, V.; Xenakis, A.; Zoumpanioti, M. Co-Encapsulation of Phycocyanin and Albumin-Bound Curcumin in Biopolymeric Hydrogels. Int. J. Mol. Sci. 2025, 26, 3805. https://doi.org/10.3390/ijms26083805
Matskou K, Matis I, Demisli S, Rigkos K, Karandrea E, Kourioti K, Sotiroudis G, Pletsa V, Xenakis A, Zoumpanioti M. Co-Encapsulation of Phycocyanin and Albumin-Bound Curcumin in Biopolymeric Hydrogels. International Journal of Molecular Sciences. 2025; 26(8):3805. https://doi.org/10.3390/ijms26083805
Chicago/Turabian StyleMatskou, Konstantina, Ilias Matis, Sotiria Demisli, Konstantinos Rigkos, Eirini Karandrea, Kalliopi Kourioti, Georgios Sotiroudis, Vasiliki Pletsa, Aristotelis Xenakis, and Maria Zoumpanioti. 2025. "Co-Encapsulation of Phycocyanin and Albumin-Bound Curcumin in Biopolymeric Hydrogels" International Journal of Molecular Sciences 26, no. 8: 3805. https://doi.org/10.3390/ijms26083805
APA StyleMatskou, K., Matis, I., Demisli, S., Rigkos, K., Karandrea, E., Kourioti, K., Sotiroudis, G., Pletsa, V., Xenakis, A., & Zoumpanioti, M. (2025). Co-Encapsulation of Phycocyanin and Albumin-Bound Curcumin in Biopolymeric Hydrogels. International Journal of Molecular Sciences, 26(8), 3805. https://doi.org/10.3390/ijms26083805







