Evaluation of Tyrosinase Inhibitory Activity of Carbathioamidopyrazoles and Their Potential Application in Cosmetic Products and Melanoma Treatment
Abstract
1. Introduction
2. Results
2.1. Molecular Docking to Tyrosinase
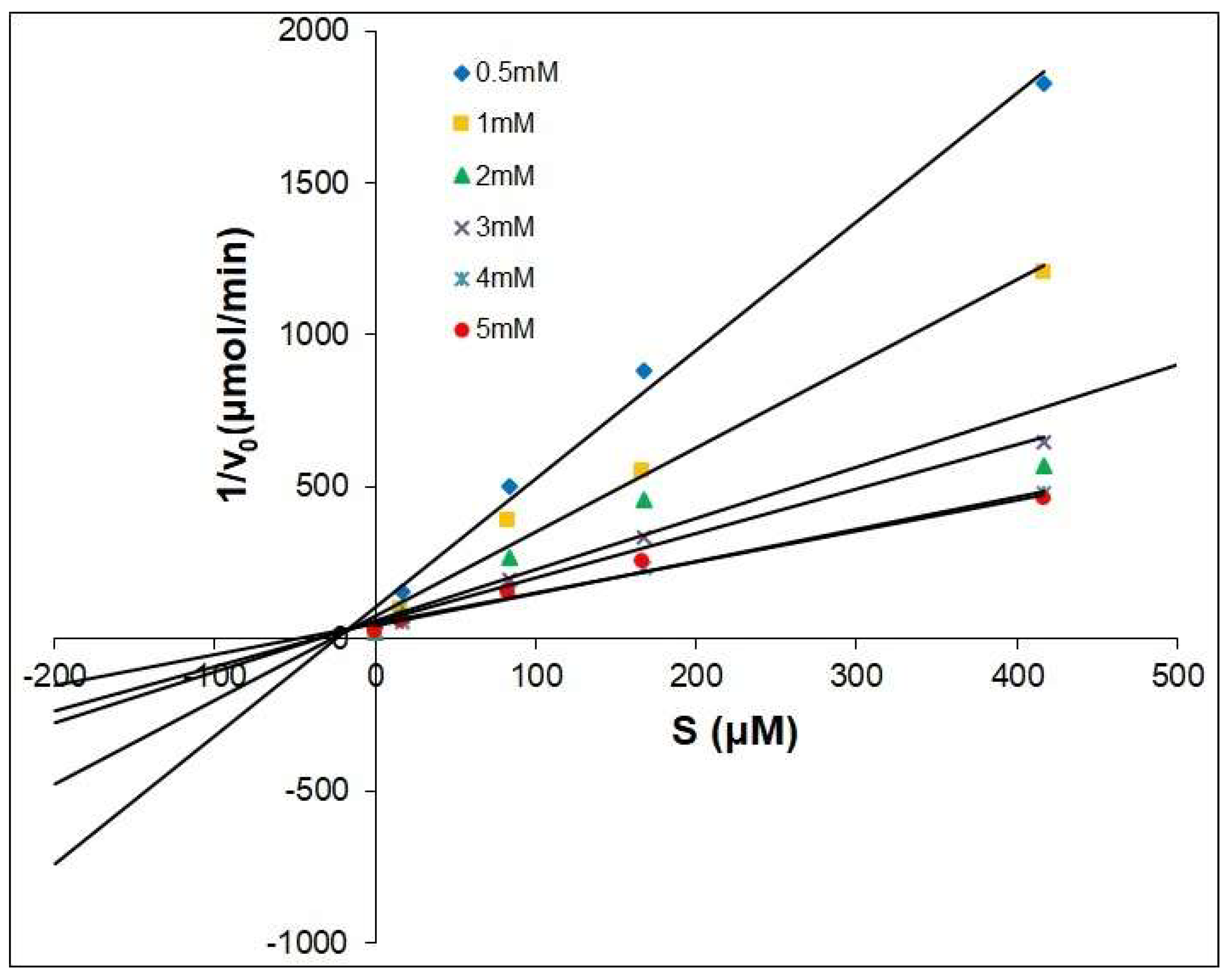
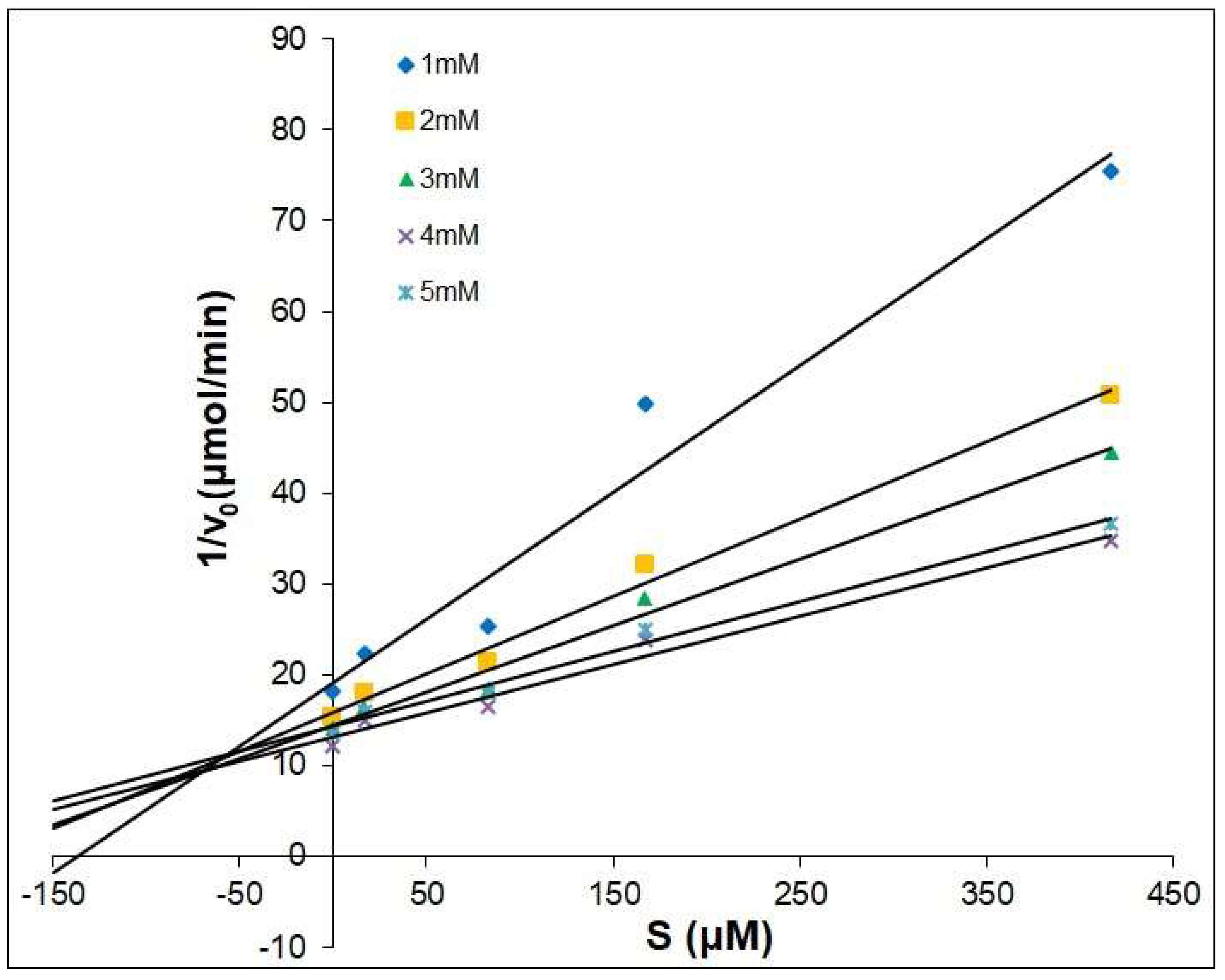
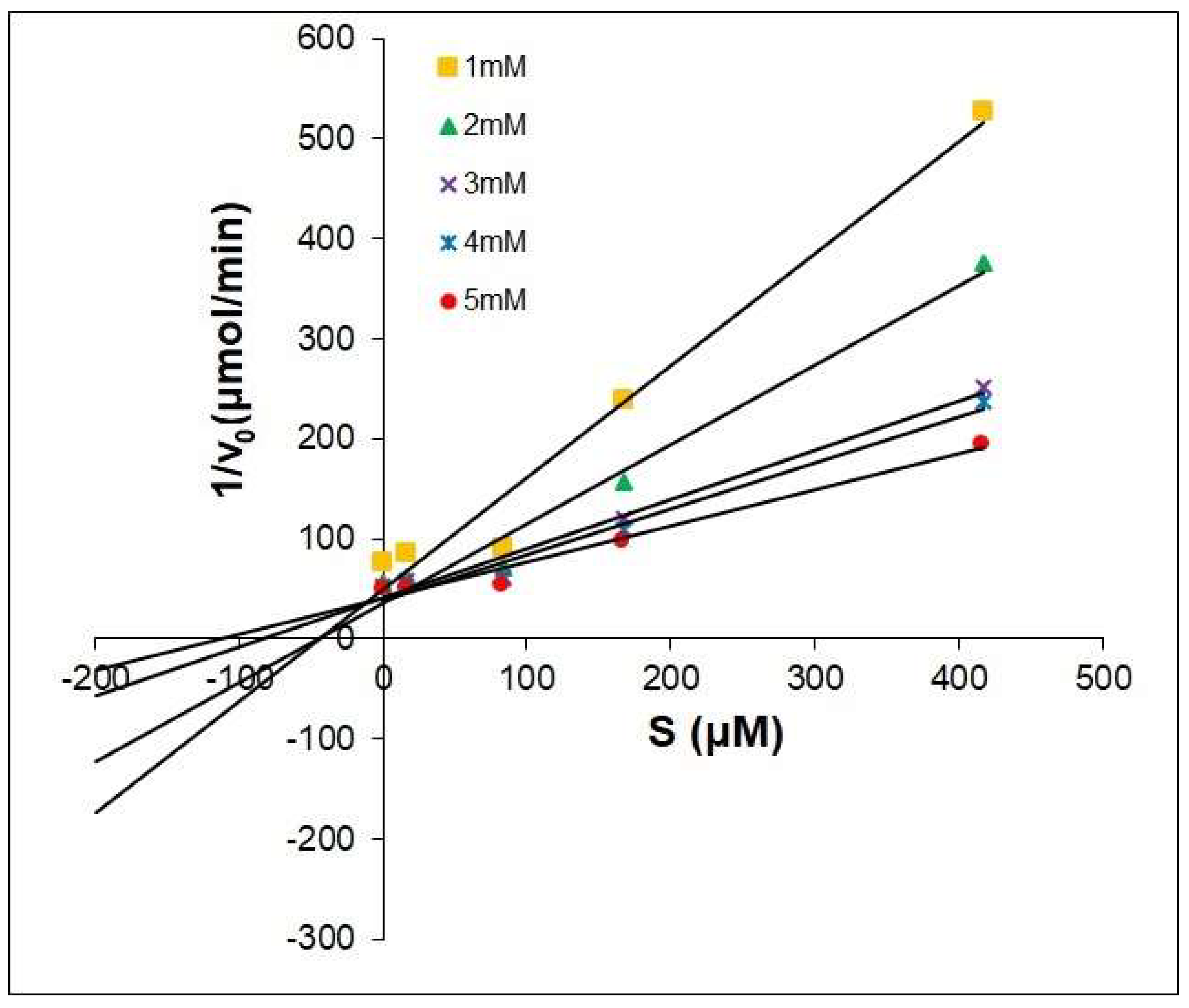
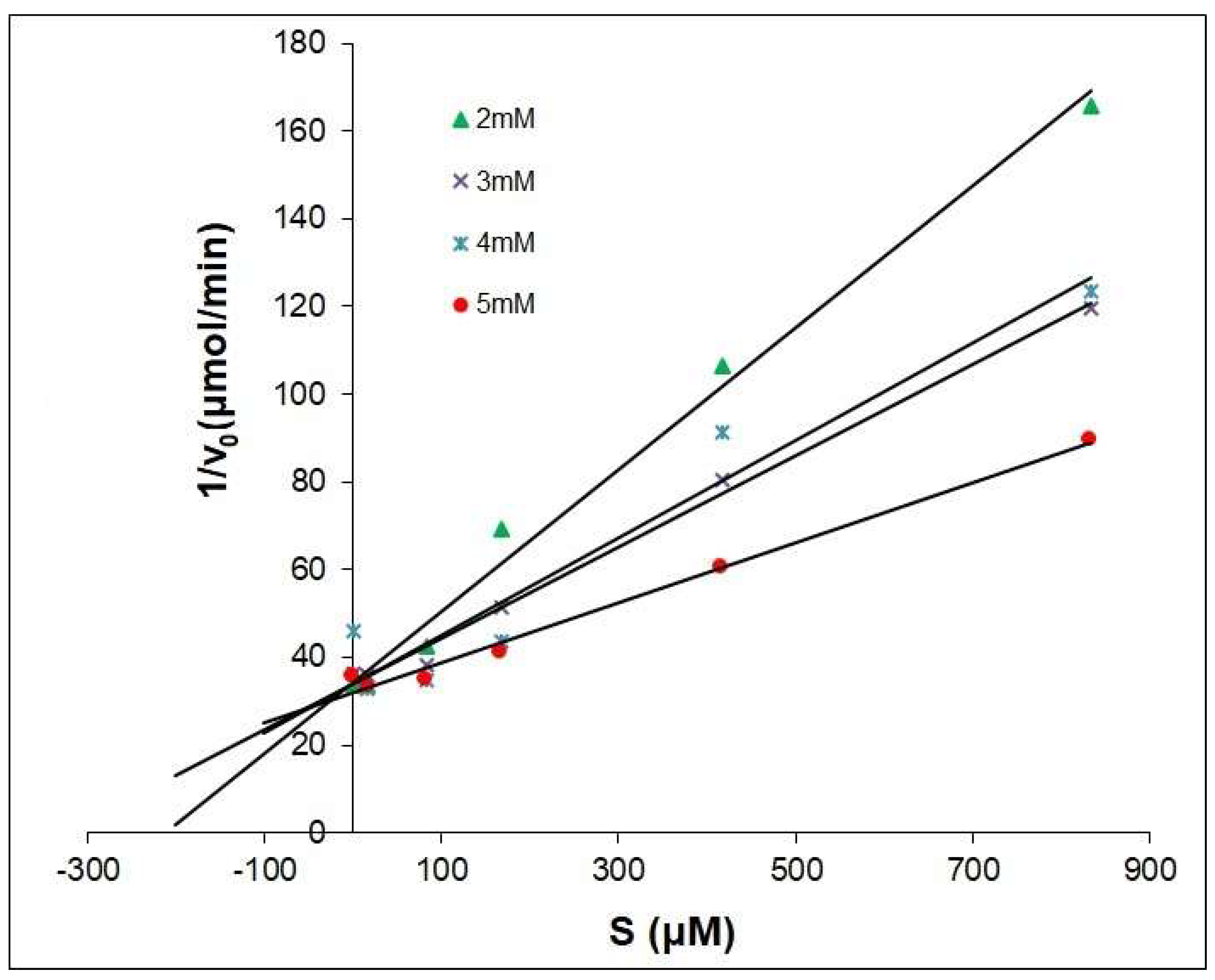
2.2. The Dixon Kinetics Test
- v—initial rate of inhibition reaction;
- V—maximum velocity obtained at high substrate concentrations;
- Km—Michaelis constant;
- Ki—Inhibition constant;
- i—concentration of inhibitor;
- s—concentration of substrate.
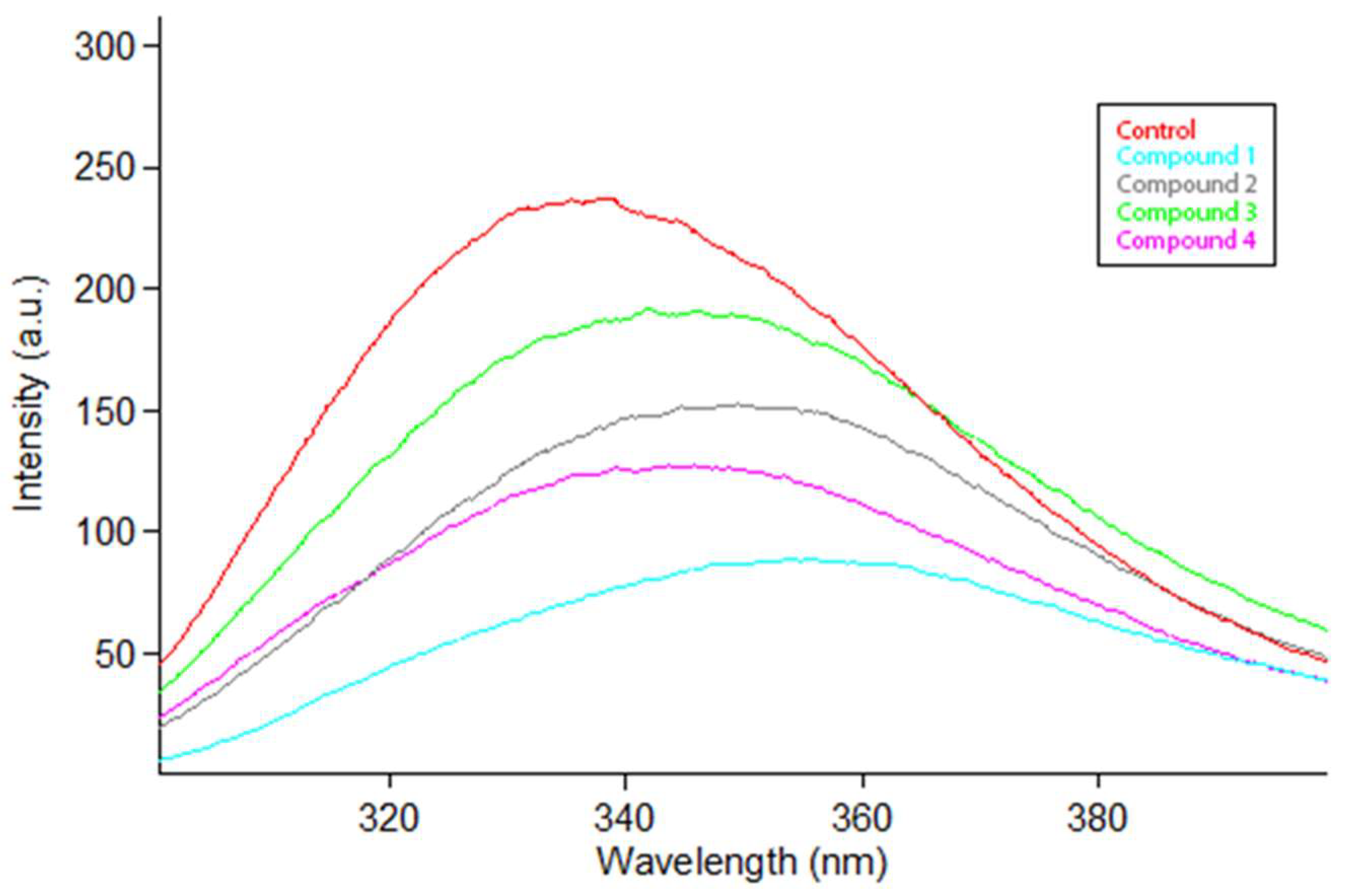
2.3. Changes in Tryptophan Fluorescence Intensity–Fluorescence Quenching Mechanism
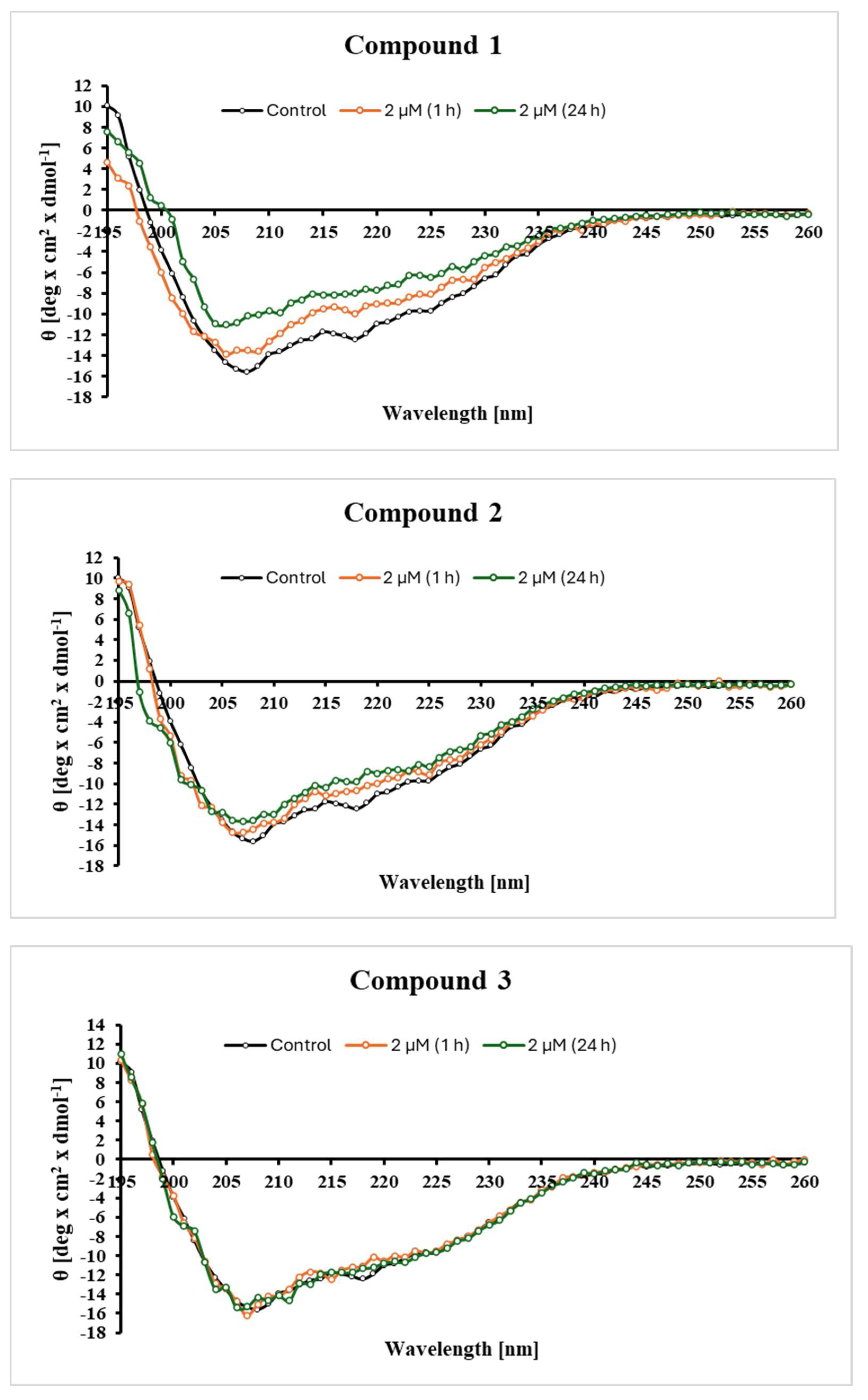
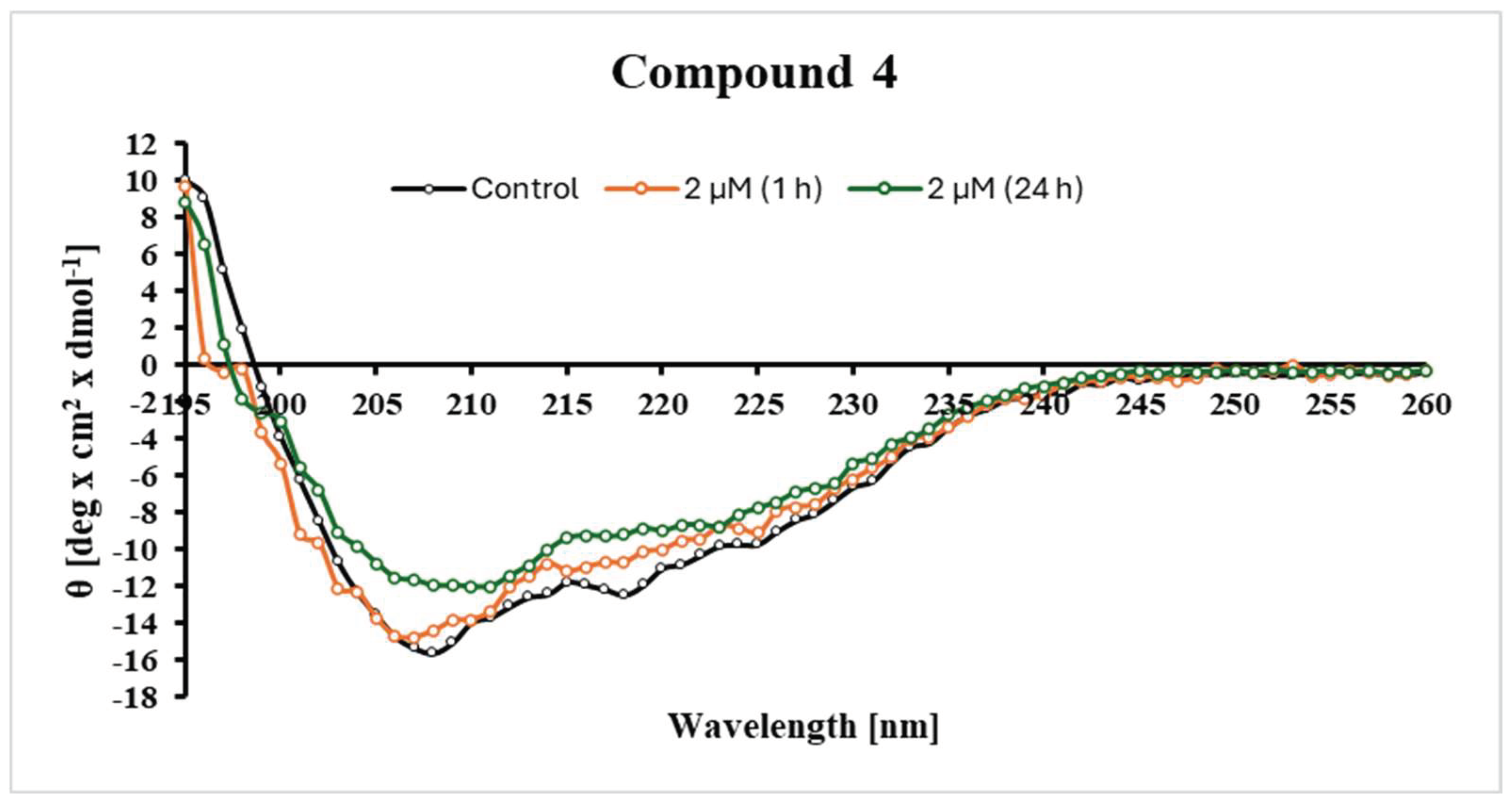
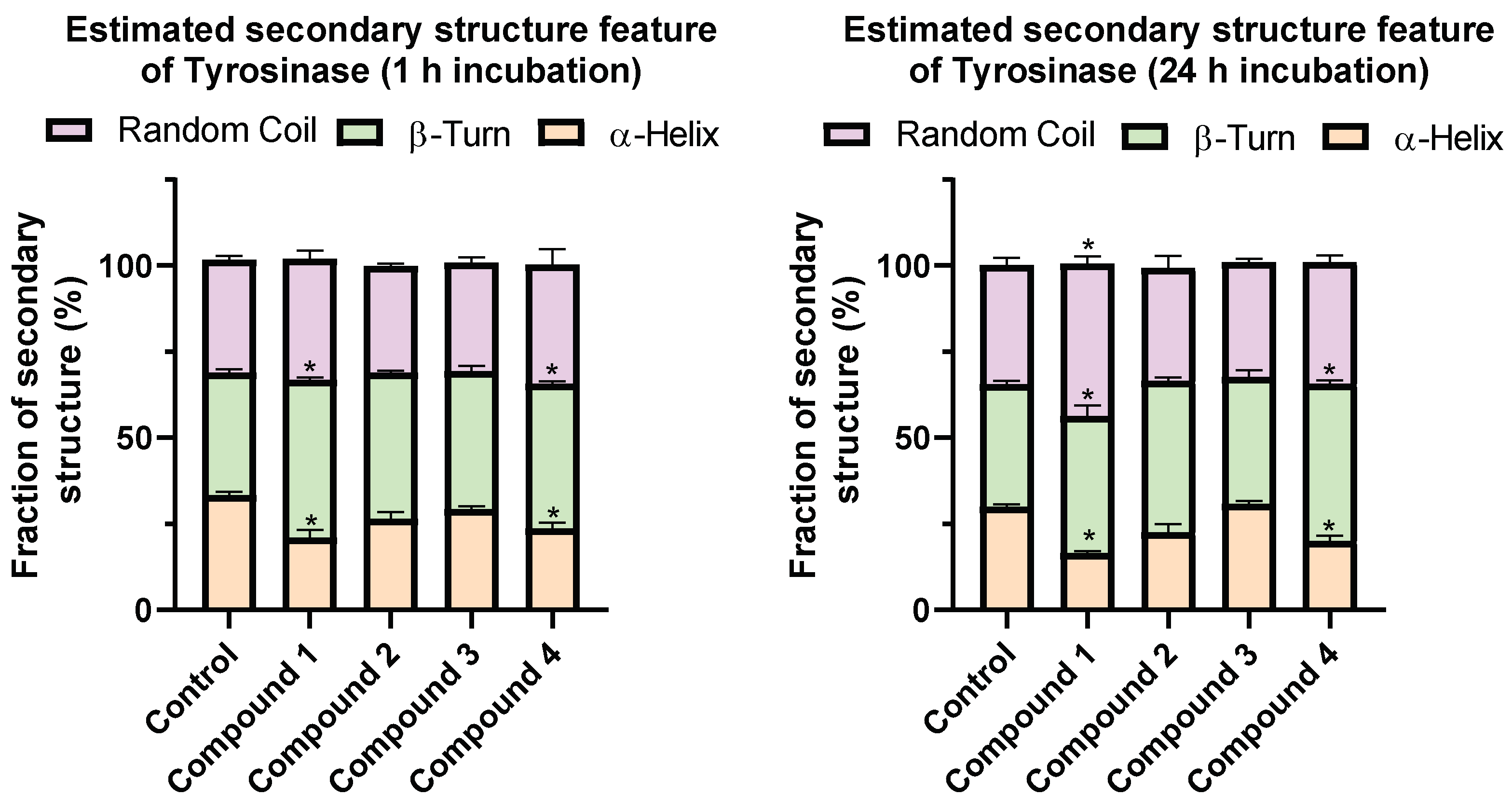
2.4. Changes in the Secondary Structure of Tyrosinase
3. Discussion
4. Experimental Section, Materials and Methods
4.1. Computational Studies
4.1.1. Ligand Preparation
4.1.2. Protein Preparation
4.1.3. Molecular Docking
4.2. The Kinetics Method of Dixon
4.3. Determination of the Secondary Structure of Tyrosinase—Circular Dichroism (CD)
4.4. Fluorescence Quenching Mechanism of Tyrosinase
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Masum, M.N.; Yamauchi, K.; Mitsunaga, T. Tyrosinase Inhibitors from Natural and Synthetic Sources as Skin-Lightening Agents. Rev. Agric. Sci. 2019, 7, 41–58. [Google Scholar] [CrossRef]
- Hearing, V.J. Determination of melanin synthetic pathways. J. Investig. Dermatol. 2011, 131, 8–11. [Google Scholar] [CrossRef]
- Hu, Y.; Zeng, H.; Jiang, J.; Yang, S.; Huang, J.; Chen, J.; Zeng, Q. Evaluation of a resorufin-based fluorescent probe for tyrosinase detection in skin pigmentation disorders. Bio-Des. Manuf. 2021, 4, 806–817. [Google Scholar] [CrossRef]
- Fustero, S.; Sánchez-Roselló, M.; Barrio, P.; Simón-Fuentes, A. From 2000 to Mid-2010: A fruitful decade for the synthesis of pyrazoles. Chem. Rev. 2011, 111, 6984–7034. [Google Scholar] [CrossRef]
- Khan, M.T.H. Novel tyrosinase inhibitors from natural resources—Their computational studies. Curr. Med. Chem. 2012, 19, 2262–2272. [Google Scholar] [CrossRef] [PubMed]
- Bao, K.; Dai, Y.; Zhu, Z.B.; Tu, F.J.; Zhang, W.G.; Yao, X.S. Design and synthesis of biphenyl derivatives as mushroom tyrosinase inhibitors. Bioorg. Med. Chem. 2010, 18, 6708–6714. [Google Scholar] [CrossRef]
- Kim, Y.J.; Uyama, H. Tyrosinase inhibitors from natural and synthetic sources: Structure, inhibition mechanism and perspective for the future. Cell. Mol. Life Sci. 2005, 62, 1707–1723. [Google Scholar] [CrossRef]
- Saeedi, M.; Eslamifar, M.; Khezri, K. Kojic acid applications in cosmetic and pharmaceutical preperations. Biomed. Pharmacother. 2019, 210, 582–593. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.S. An updated review of tyrosinase inhibitors. Int. J. Mol. Sci. 2009, 10, 2440–2475. [Google Scholar] [CrossRef]
- Maeda, K.; Fukuda, M. In vitro effectiveness of several whitening cosmetic components in human melanocytes. J. Soc. Cosmet. Chem. 1991, 42, 361–368. [Google Scholar]
- Jimbow, K.; Obata, H.; Pathak, M.A.; Fitzpatrick, T.B. Mechanism of depigmentation by hydroquinone. J. Investig. Dermatol. 1974, 62, 436–449. [Google Scholar] [CrossRef] [PubMed]
- Curto, E.V.; Kwong, C.; Hermersdörfer, H.; Glatt, H.; Santis, C.; Virador, V.; Hearing, V.J.; Dooley, T.P. Inhibitors of mammalian melanocyte tyrosinase: In vitro comparisons of alkyl esters of gentisic acid with other putative inhibitors. Biochem. Pharmacol. 1999, 57, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Gillbro, J.; Olsson, M. The melanogenesis and mechanisms of skin-lightening agents-existing and new approaches. Int. J. Cosmet. Sci. 2011, 33, 210–221. [Google Scholar] [CrossRef] [PubMed]
- Orqueda, M.E.; Zampini, I.C.; Bravo, K.; Osorio, E.; Isla, M.I. Potential use of native fruits waste from Argentina as nonconventional sources of cosmetic ingredients. J. Cosmet. Dermatol. 2022, 21, 5058–5065. [Google Scholar] [CrossRef]
- Muruganandam, A.R.; Venkatasubramanian, S.; Jagmag, S.A.; Veerichetty, V. Antityrosinase Activity of Phycocyanin and Cream Formulation for Hyperpigmentation. IOP Conf. Ser. Mater. Sci. Eng. 2023, 1291, 12039. [Google Scholar] [CrossRef]
- Kang, M.H.; Jang, G.Y.; Ji, Y.-J.; Lee, J.H.; Choi, S.J.; Hyun, T.K.; Kim, H.D. Antioxidant and Anti-Melanogenic Activities of Heat-Treated Licorice (Wongam, Glycyrrhiza glabra × G. uralensis) Extract. Curr. Issues Mol. Biol. 2021, 43, 1171–1187. [Google Scholar] [CrossRef]
- Kim, Y.M.; Yun, J.; Lee, C.-K.; Lee, H.; Min, K.R.; Kim, Y. Oxyresveratrol and Hydroxystilbene Compounds. Biol. Pharm. Bull. 2002, 25, 1045–1048. [Google Scholar] [CrossRef]
- Kaur, C.; Kapoor, H.C. Anti-oxidant activity and total phenolic content of some Asian vegetables. Int. J. Food Sci. Technol. 2002, 37, 153–161. [Google Scholar] [CrossRef]
- Hassan, M.; Shahzadi, S.; Kloczkowski, A. Tyrosinase Inhibitors Naturally Present in Plants and Synthetic Modifications of These Natural Products as Anti-Melanogenic Agents: A Review. Molecules 2023, 28, 378. [Google Scholar] [CrossRef]
- Logesh, R.; Prasad, S.R.; Chipurupalli, S.; Robinson, N.; Mohankumar, S.K. Natural tyrosinase enzyme inhibitors: A path from melanin to melanoma and its reported pharmacological activities. Biochim. Biophys. Acta (BBA)—Rev. Cancer 2023, 1878, 188968. [Google Scholar] [CrossRef]
- Pillaiyar, T.; Manickam, M.; Namasivayam, V. Skin whitening agents: Medicinal chemistry perspective of tyrosinase inhibitors. J. Enzym. Inhib. Med. Chem. 2017, 32, 403–425. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.J.S.; Yang, J.C.; Chu, F.H.; Chang, S.T.; Wang, S.Y. Lucidone, a novel melanin inhibitor from the fruit of Lindera erythrocarpa Makino. Phytother. Res. 2010, 24, 1158–1165. [Google Scholar] [CrossRef]
- Hwang, Y.; Lee, J.; Jung, H.J.; Ullah, S.; Ko, J.; Jeong, Y.; Park, Y.J.; Kang, M.K.; Yun, H.; Kim, M.-S.; et al. A Novel Class of Potent Anti-Tyrosinase Compounds with Antioxidant Activity, 2-(Substituted phenyl)-5-(trifluoromethyl)benzo[d]thiazoles: In Vitro and In Silico Insights. Antioxidants 2022, 11, 1375. [Google Scholar] [CrossRef] [PubMed]
- Hariri, R.; Saeedi, M.; Akbarzadeh, T. Naturally occurring and synthetic peptides: Efficient tyrosinase inhibitors. J. Pept. Sci. 2021, 27, 3329. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.S.; Chao, S.Y.; Ding, H.Y. Melanogenesis inhibition by homoisoflavavone sappanone A from Caesalpinia sappan. Int. J. Mol. Sci. 2012, 13, 10359–10367. [Google Scholar] [CrossRef]
- Beltran, E.; Serafini, M.R.; Alves, I.A.; Novoa, D.M.A. Novel Synthesized Tyrosinase Inhibitors: A Systematic Patent Review (2012–Present). Curr. Med. Chem. 2024, 31, 308–335. [Google Scholar] [CrossRef]
- Namiecinska, E.; Sadowska, B.; Wieckowska-Szakiel, M.; Dołega, A.; Pasternak, B.; Grazul, M.; Budzisz, E. Anticancer and antimicrobial properties of novel h6-p-cymene ruthenium(II) complexes containing a N,S-type ligand, their structural and theoretical characterization. RSC Adv. 2019, 9, 38629–38645. [Google Scholar] [CrossRef] [PubMed]
- Ghani, U.; Ullah, N. New potent inhibitors of tyrosinase: Novel clues to binding of 1,3,4-thiadiazole-2(3H)-thiones, 1,3,4-oxadiazole-2(3H)-thiones, 4-amino-1,2,4-triazole-5(4H)-thiones, and substituted hydrazides to the dicopper active site. Bioorg. Med. Chem. 2010, 18, 4042–4048. [Google Scholar] [CrossRef]
- Peng, Z.; Wang, G.; Zeng, Q.-H.; Li, Y.; Liu, H.; Wang, J.J. A systematic review of synthetic tyrosinase inhibitors and their structure–activity relationship. Crit. Rev. Food Sci. Nutr. 2022, 62, 4053–4094. [Google Scholar] [CrossRef]
- Burlingham, B.T.; Wilanski, T.S. An Intuitive Look at the Relationship of Ki and IC50: A More General Use for the Dixon Plot. Concepts Biochem. 2003, 80, 214–218. [Google Scholar] [CrossRef]
- Dixon, M. The Determination of Enzyme Inhibitor Constants. Biochem. J. 1953, 55, 170–171. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Rao, J.; Wang, K.; Wang, M.; Yao, T.; Qiu, F. Highly potent inhibition of tyrosinase by mulberrosides and the inhibitory mechanism in vitro. Chem. Biodivers. 2021, 19, e202100740. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Chen, R.; Fan, Y.; Liu, G.; Wang, M.; Wang, S.; Li, L. Interaction mechanism of Pelargonidin against tyrosinase by multi-spectroscopy and molecular docking. J. Mol. Recognit. 2022, 35, e2955. [Google Scholar] [CrossRef]
- Anantharaman, A.; Hemachandran, H.; Priya, R.R.; Sankari, M.; Gopalakrishnan, M.; Palanisami, N.; Siva, R. Inhibitory effect of apocarotenoids on the activity of tyrosinase: Multi-spectroscopic and Docking Studies. J. Biosci. Bioeng. 2016, 121, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Li, Y.; Guo, J.; Sun, W.; Lv, Y. Synthetic polymer nanoparticles as an abiotic artificial inhibitor of tyrosinase. Adv. Healthc. Mater. 2024, 13, 2303615. [Google Scholar] [CrossRef]
- Baber, M.A.; Crist, C.M.; Devolve, N.L.; Patrone, J.D. Tyrosinase Inhibitors: A Perspective. Molecules 2023, 28, 5762. [Google Scholar] [CrossRef]
- Vargas, A.J.; Sittadjody, S.; Thangasamy, T.; Mendoza, E.E.; Limesand, K.H.; Burd, R. Exploiting tyrosinase expression and activity in melanocytic tumors. Integr. Cancer Ther. 2011, 10, 328–340. [Google Scholar] [CrossRef]
- Hałdys, K.; Goldeman, W.; Jewgiński, M.; Wolińska, E.; Anger-Góra, N.; Rossowska, J.; Latajka, R. Halogenated Aromatic Thiosemicarbazones as Potent Inhibitors of Tyrosinase and Melanogenesis. Bioorg. Chem. 2020, 94, 103419. [Google Scholar] [CrossRef]
- Bochot, C.; Gouron, A.; Bubacco, L.; Milet, A.; Philouze, C.; Réglier, M.; Serratrice, G.; Jamet, H.; Belle, C. Probing kojic acid binding to tyrosinase enzyme: Insights from a model complex and QM/MM calculations. Chem. Commun. 2014, 50, 308–310. [Google Scholar] [CrossRef]
- Hikisz, P.; Namiecińska, E.; Paneth, P.; Budzisz, E. Mechanistic Studies of Arene–Ruthenium(II) Complexes with Carbothioamidopyrazoles as Alternative Cancer Drugs. Molecules 2023, 28, 3969. [Google Scholar] [CrossRef]
- Kamiński, K.; Kazimierczak, U.; Kolenda, T. Oxidative stress in melanogenesis and melanoma development. Contemp. Oncol. 2022, 26, 1–7. [Google Scholar] [CrossRef]
- Shi, X.; Chang, Z.; Wu, X.; Zhang, G.; Peng, Z.; Dong, Z.; Shao, X. Inactivation effect of argon atmospheric pressure low-temperature plasma jet on murine melanoma cells. Plasma Process. Polym. 2013, 10, 808–816. [Google Scholar] [CrossRef]
- Wang, W.; Yang, D.; Zhou, Y.; Zhang, Y.; Guan, L.; Zhang, X.; Xue, W.; Huang, S. Novel green fluorescent probe stem from carbon quantum dots for specific recognition of tyrosinase in serum and Living Cells. J. Fluoresc. 2022, 33, 739–750. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Wang, T.; Li, G.; Huang, J.; Yuan, Q. Dual-locked near-infrared fluorescent probes for precise detection of melanoma via hydrogen peroxide–tyrosinase cascade activation. Anal. Chem. 2021, 94, 1070–1075. [Google Scholar] [CrossRef]
- Kurt, B.Z.; Altundağ, Ö.; Gökçe, M.; Cakmak, U.; Tuncay, F.O.; Kolcuoğlu, Y.; Akyıldız, A.G.; Akdemir, A.; Civelek, D.Ö.; Sönmez, F. Synthesis of naproxen thiadiazole urea hybrids and determination of their anti-melanoma, anti-migration, tyrosinase inhibitory activity, and Molecular Docking Studies. J. Mol. Struct. 2024, 1295, 136618. [Google Scholar] [CrossRef]
- Roulier, B.; Rush, I.; Lazinski, L.M.; Pérès, B.; Olleik, H.; Royal, G.; Fishman, A.; Maresca, M.; Haudecoeur, R. Resorcinol-based hemiindigoid derivatives as human tyrosinase inhibitors and melanogenesis suppressors in human melanoma cells. Eur. J. Med. Chem. 2023, 246, 114972. [Google Scholar] [CrossRef]
- Goenka, S.; Johnson, F.; Simon, S.R. Novel chemically modified curcumin (CMC) derivatives inhibit tyrosinase activity and melanin synthesis in B16F10 mouse melanoma cells. Biomolecules 2021, 11, 674. [Google Scholar] [CrossRef] [PubMed]
- Azimi, F.; Mahdavi, M.; Khoshneviszadeh, M.; Shafiee, F.; Azimi, M.; Hassanzadeh, F.; Ashrafee, F.H. Kinetic Studies, molecular docking, and antioxidant activity of novel 1,3-diphenyl pyrazole-thiosemicarbazone with anti-tyrosinase and anti-melanogenesis properties. Bioorg. Chem. 2024, 152, 107722. [Google Scholar] [CrossRef]
- Boateng, S.T.; Roy, T.; Torrey, K.; Owunna, U.; Banang-Mbeumi, S.; Basnet, D.; Niedda, E.; Alexander, A.D.; El Hage, D.; Atchimnaidu, S.; et al. Synthesis, in silico modelling, and in vitro biological evaluation of substituted pyrazole derivatives as potential anti-skin cancer, anti-tyrosinase, and antioxidant agents. J. Enzym. Inhib. Med. Chem. 2023, 38, 2205042. [Google Scholar] [CrossRef]
- Nasab, N.H.; Raza, H.; Eom, Y.S.; Hassan, M.; Kloczkowski, A.; Kim, S.J. Synthesis and discovery of potential tyrosinase inhibitor of new coumarin-based thiophenyl-pyrazolylthiazole nuclei: In vitro evaluation, cytotoxicity, kinetic, and computational studies. Chem. Biol. Drug Des. 2023, 101, 1262–1272. [Google Scholar]
- Mojzych, M.; Tarasiuk, P.; Kotwica-Mojzych, K.; Rafiq, M.; Seo, S.-Y.; Nicewicz, M.; Fornal, E. Synthesis of chiral pyrazolo[4,3-e][1,2,4]triazine sulfonamides with tyrosinase and urease inhibitory activity. J. Enzym. Inhib. Med. Chem. 2016, 32, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Iervasi, E.; Vargas, G.C.; Bachetti, T.; Tkachenko, K.; Spallarossa, A.; Brullo, C.; Rosano, C.; Carta, S.; Barboro, P.; Profumo, A.; et al. A proteomics approach identifies RREB1 as a crucial molecular target of imidazo–pyrazole treatment in SKMEL-28 melanoma cells. Int. J. Mol. Sci. 2024, 25, 6760. [Google Scholar] [CrossRef] [PubMed]
- Taherkhani, N.; Hekmat, A.; Piri, H.; Haghbeen, K. Structural and inhibitory effects of fulvic and humic acids against tyrosinase. J. Food Biochem. 2022, 46, e14279. [Google Scholar] [CrossRef]
- Liu, W.; You, X.; Wang, C.; Guo, N.; Desk, S. Study on the interaction of kojic acid with tyrosinase by spectroscopic methods. J. Comput. Chem. Mol. Model. 2020, 4, 365–375. [Google Scholar] [CrossRef]
- Li, W.; Tian, H.; Guo, F.; Wu, Y. Inhibition characteristics and mechanism of tyrosinase using five citrus flavonoids: A spectroscopic and Molecular Dynamics Simulation Study. J. Food Biochem. 2022, 46, e14484. [Google Scholar] [CrossRef]
- Vaezi, M. Evaluation of quercetin omega-6 and -9 esters on activity and structure of mushroom tyrosinase: Spectroscopic and molecular docking studies. J. Food Biochem. 2021, 45, e13953. [Google Scholar] [CrossRef]
- Zhang, S.; Ai, H. A general strategy to red-shift green fluorescent protein-based biosensors. Nat. Chem. Biol. 2020, 16, 1434–1439. [Google Scholar] [CrossRef]
- Cui, Y.; Hu, Y.-H.; Yu, F.; Zheng, J.; Chen, L.-S.; Chen, Q.-X.; Wang, Q. Inhibition kinetics and molecular simulation of P-substituted cinnamic acid derivatives on tyrosinase. Int. J. Biol. Macromol. 2017, 95, 1289–1297. [Google Scholar] [CrossRef]
- You, A.; Zhou, J.; Song, S.; Zhu, G.; Song, H.; Yi, W. Rational design, synthesis and structure–activity relationships of 4-alkoxy- and 4-acyloxy-phenylethylenethiosemicarbazone analogues as novel tyrosinase inhibitors. Bioorg. Med. Chem. 2015, 23, 924–931. [Google Scholar] [CrossRef]
- Pei, Y.; Yuan, L.; Zhou, W.; Yang, J. Tyrosinase-catalyzed soy protein and tannic acid interaction: Effects on structural and rheological properties of complexes. Gels 2025, 11, 195. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, X.; Ni, H.; Du, X.; Chen, F.; Jiang, Z.; Li, Q. Identification and characterization of the tyrosinase inhibitory activity of caffeine from camellia pollen. J. Agric. Food Chem. 2019, 67, 12741–12751. [Google Scholar] [CrossRef]
- Kahriman, N.; Haşimoğlu, Z.; Serdaroğlu, V.; Beriş, F.Ş.; Barut, B.; Yaylı, N. Synthesis of novel pyrazolines, their boron–fluorine complexes, and investigation of antibacterial, antioxidant, and enzyme inhibition activities. Arch. Pharm. 2016, 350, 1600285. [Google Scholar] [CrossRef] [PubMed]
- Briganti, S.; Camera, E.; Picardo, M. Chemical and Instrumental Approaches to Treat Hyperpigmentation. Pigment. Cell Res. 2003, 16, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Solano, F. Melanins: Skin Pigments and Much More—Types, Structural Models, Biological Functions, and Formation Routes. New J. Sci. 2014, 2014, 498276. [Google Scholar] [CrossRef]
- Zolghadri, S.; Bahrami, A.; Khan, M.T.H.; Munoz-Munoz, J.; Garcia-Molina, F.; Garcia-Canovas, F.; Saboury, A.A. A Comprehensive Review on Tyrosinase Inhibitors. J. Enzym. Inhib. Med. Chem. 2019, 34, 279–309. [Google Scholar] [CrossRef]
- Mann, T.; Gerwat, W.; Batzer, J.; Eggers, K.; Scherner, C.; Wenck, H.; Stäb, F.; Röhm, K.H.; Kolbe, L. Inhibition of Human Tyrosinase Requires Molecular Specificity. J. Investig. Dermatol. 2018, 138, 1601–1608. [Google Scholar] [CrossRef]
- Yu, R.J.; Van Scott, E.J. Alpha-Tocopheryl Glucoside and Deoxyarbutin for Skin Lightening. J. Cosmet. Dermatol. 2009, 8, 214–223. [Google Scholar] [CrossRef]
- Sugimoto, K.; Nishimura, T.; Nomura, K.; Sugimoto, K. Comparison of the Effects of α-Arbutin and Arbutin on Melanin Biosynthesis in Cultured Human Melanocytes. Biol. Pharm. Bull. 2004, 27, 510–514. [Google Scholar] [CrossRef]
- Parvez, S.; Kang, M.; Chung, H.S.; Bae, H. Naturally Occurring Tyrosinase Inhibitors: Mechanism and Applications in Skin Health, Cosmetics and Agriculture Industries. Phytother. Res. 2007, 21, 805–816. [Google Scholar] [CrossRef]
- Kong, Y.; Zhang, L.; Guo, Y.; Wang, Y.; Wang, M. Tyrosinase Inhibitory Mechanisms of Glabridin: A Review. Molecules 2019, 24, 2740. [Google Scholar] [CrossRef]
- Chawla, S.; Kvalnes, K.; Delong, M.A.; Wickett, R.; Manga, P.; Boissy, R.E. DeoxyArbutin and Its Derivatives Inhibit Tyrosinase Activity and Melanin Synthesis Without Inducing Reactive Oxygen Species or Apoptosis. J. Drugs Dermatol. 2012, 11, e28–e34. [Google Scholar] [PubMed]
- Boissy, R.E.; Visscher, M.; DeLong, M.A. DeoxyArbutin: A Novel Reversible Tyrosinase Inhibitor with Effective In Vivo Skin Lightening Potency. Exp. Dermatol. 2005, 14, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Grazul, M.; Besic-Gyenge, E.; Maake, C.; Ciolkowski, M.; Czyz, M.; Sigel, R.K.; Budzisz, E. Synthesis, physico-chemical properties and biological analysis of newly obtained copper(II) complexes with pyrazole derivatives. J. Inorg. Biochem. 2014, 135, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Sobiesiak, M.; Muzioł, T.; Rozalski, M.; Krajewska, U.; Budzisz, E. Co(II), Ni(II) and Cu(II) complexes with phenylthiazole and thiosemicarbazone-derived ligands: Synthesis, structure and cytotoxic effects. New J. Chem. 2014, 38, 5349–5361. [Google Scholar] [CrossRef]
- Sobiesiak, M.; Cieślak, M.; Krolewska, K.; Kaźmierczak-Barańska, J.; Pasternak, B.; Budzisz, E. Thiosemicarbazone-derived copper(II), cobalt(II) and nickel(II) complexes as potential anticancer agents: Nuclease activity, cytotoxicity and apoptosis studies. New J. Chem. 2016, 40, 9761–9767. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. Software news and update AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Chen, J.; Ye, Y.; Ran, M.; Li, Q.; Ruan, Z.; Jin, N. Inhibition of tyrosinase by mercury chloride: Spectroscopic and docking studies. Front. Pharmacol. 2020, 11, 81. [Google Scholar] [CrossRef]
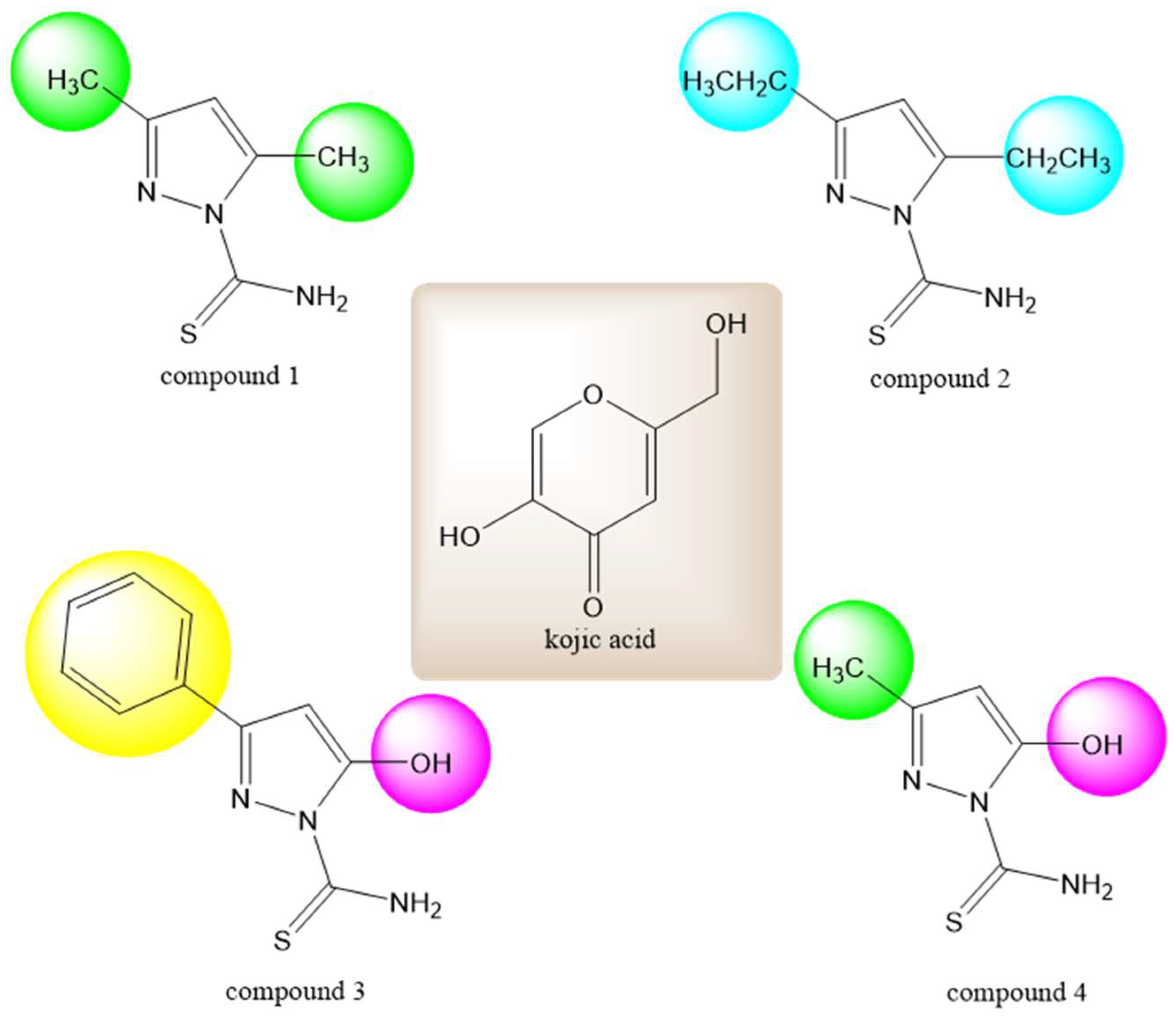

| Name | Structure | Free Energy of Binding [kcal/mol] |
|---|---|---|
| Compound 1 |  | −5.2 |
| Compound 2 | 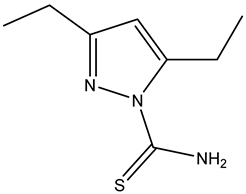 | −5.3 |
| Compound 3 | 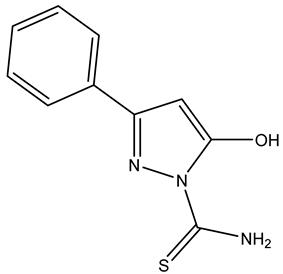 | −2.1 |
| Compound 4 |  | −5.4 |
| Kojic acid |  | −5.7 |
| Name | The Binding Mode to Tyrosinase | Residue and Distance (Å) |
|---|---|---|
| Compound 1—red Compound 2—blue Compound 3—yellow Compound 4—green Kojic acid—orange |  | - |
| Compound 1 | 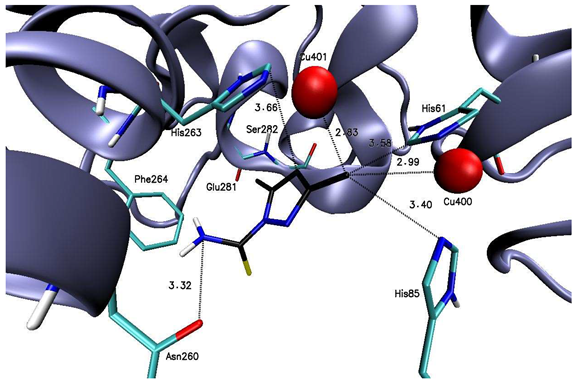 | His61 (3.58), His85 (3.40), Asn260 (3.32), His263 (3.66), Cu400 (2.99), Cu401 (2.83) |
| Compound 2 |  | His61 (3.46), His85 (3.39), Glu256 (3.20), Asn260 (2.88, 3.39), Phe264 (3.68) Cu400 (3.26), Cu401 (2.88) |
| Compound 3 |  | His85 (3.00), Glu256 (2.98), His259 (3.61), His263 (3.25), Phe264 (3.29), Cu400 (2.53), Cu401 (2.81) |
| Compound 4 |  | His259 (3.58), His263 (3.56, 3.63), Gly281 (2.96, 3.32), Cu400 (2.98), Cu401 (2.97) |
| Kojic acid | 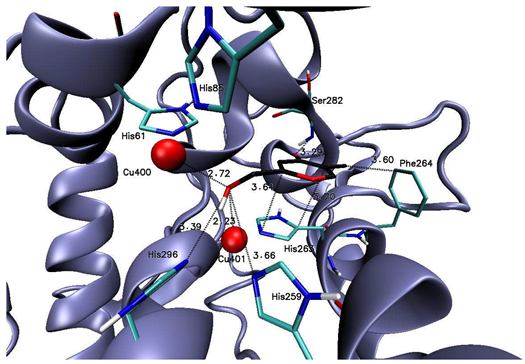 | His259 (3.66), His263 (3.64, 3.70), Phe264 (3.60), Ser282 (3.29), His296 (3.39), Cu400 (2.72), Cu401 (2.23) |
| Compounds | Type | Ki μM |
|---|---|---|
| Kojic Acid | competitive | 25.41 |
| 1 | competitive | 14.64 |
| 2 | competitive | 64.43 |
| 3 | competitive | 117.72 |
| 4 | competitive | 7.88 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Namiecińska, E.; Jaszczak, J.; Hikisz, P.; Daśko, M.; Woźniczka, M.; Budzisz, E. Evaluation of Tyrosinase Inhibitory Activity of Carbathioamidopyrazoles and Their Potential Application in Cosmetic Products and Melanoma Treatment. Int. J. Mol. Sci. 2025, 26, 3882. https://doi.org/10.3390/ijms26083882
Namiecińska E, Jaszczak J, Hikisz P, Daśko M, Woźniczka M, Budzisz E. Evaluation of Tyrosinase Inhibitory Activity of Carbathioamidopyrazoles and Their Potential Application in Cosmetic Products and Melanoma Treatment. International Journal of Molecular Sciences. 2025; 26(8):3882. https://doi.org/10.3390/ijms26083882
Chicago/Turabian StyleNamiecińska, Ewelina, Jan Jaszczak, Paweł Hikisz, Mateusz Daśko, Magdalena Woźniczka, and Elzbieta Budzisz. 2025. "Evaluation of Tyrosinase Inhibitory Activity of Carbathioamidopyrazoles and Their Potential Application in Cosmetic Products and Melanoma Treatment" International Journal of Molecular Sciences 26, no. 8: 3882. https://doi.org/10.3390/ijms26083882
APA StyleNamiecińska, E., Jaszczak, J., Hikisz, P., Daśko, M., Woźniczka, M., & Budzisz, E. (2025). Evaluation of Tyrosinase Inhibitory Activity of Carbathioamidopyrazoles and Their Potential Application in Cosmetic Products and Melanoma Treatment. International Journal of Molecular Sciences, 26(8), 3882. https://doi.org/10.3390/ijms26083882






