Rehmanniae Radix Praeparata in Blood Deficiency Syndrome: UPLC-Q-TOF-MS Profiling, Network Pharmacology, and PI3K-AKT Activation
Abstract
1. Introduction
2. Results
2.1. Analysis of Chemical Constituents
2.1.1. Identification of Chemical Constituents of RR and RRP
2.1.2. Identification of the Chemical Constituents of Rehmannia glutinosa
2.1.3. Comparative Analysis of the Chemical Constituents of Rehmannia glutinosa Before and After Processing
2.2. Results of Network Pharmacology Analysis
2.2.1. Target Analysis of Rehmanniae Radix Praeparata and Blood Deficiency Syndrome
2.2.2. Construction of a “Component-Target” Network
2.2.3. Construction of Protein–Protein Interaction (PPI) Networks
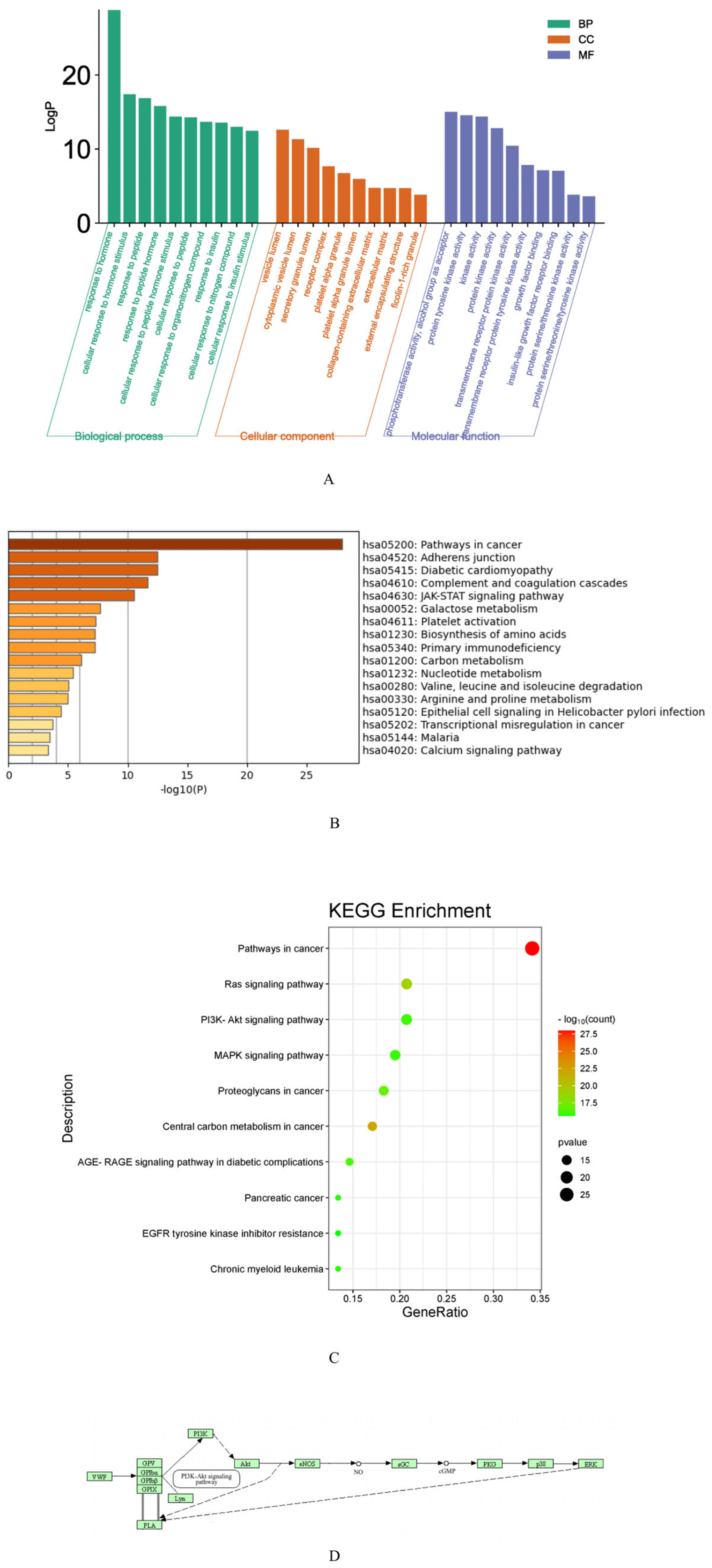
2.2.4. Kyoto Encyclopedia of Genes and Genomes and Gene Ontology Enrichment Analysis
2.2.5. Molecular Docking
2.3. Behavioral Analysis of Mice
2.3.1. Grab Test
2.3.2. Hair Status Score
2.3.3. Dynamic Changes of Body Weight
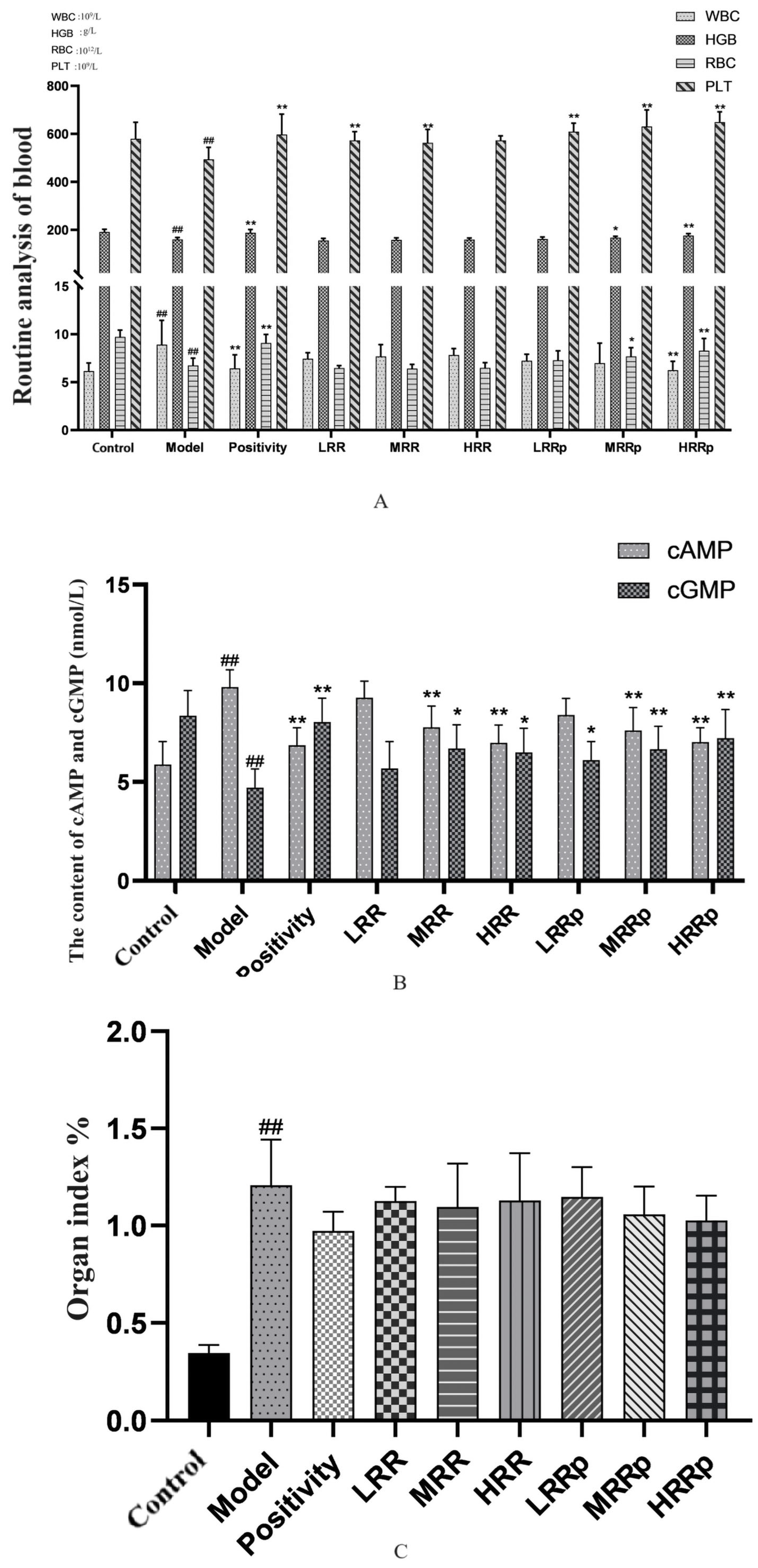
2.4. Routine Blood Testing
2.5. Organ Index
2.6. Bone Marrow, Spleen, and Abdominal Aortic Vascular H&E Staining Observation
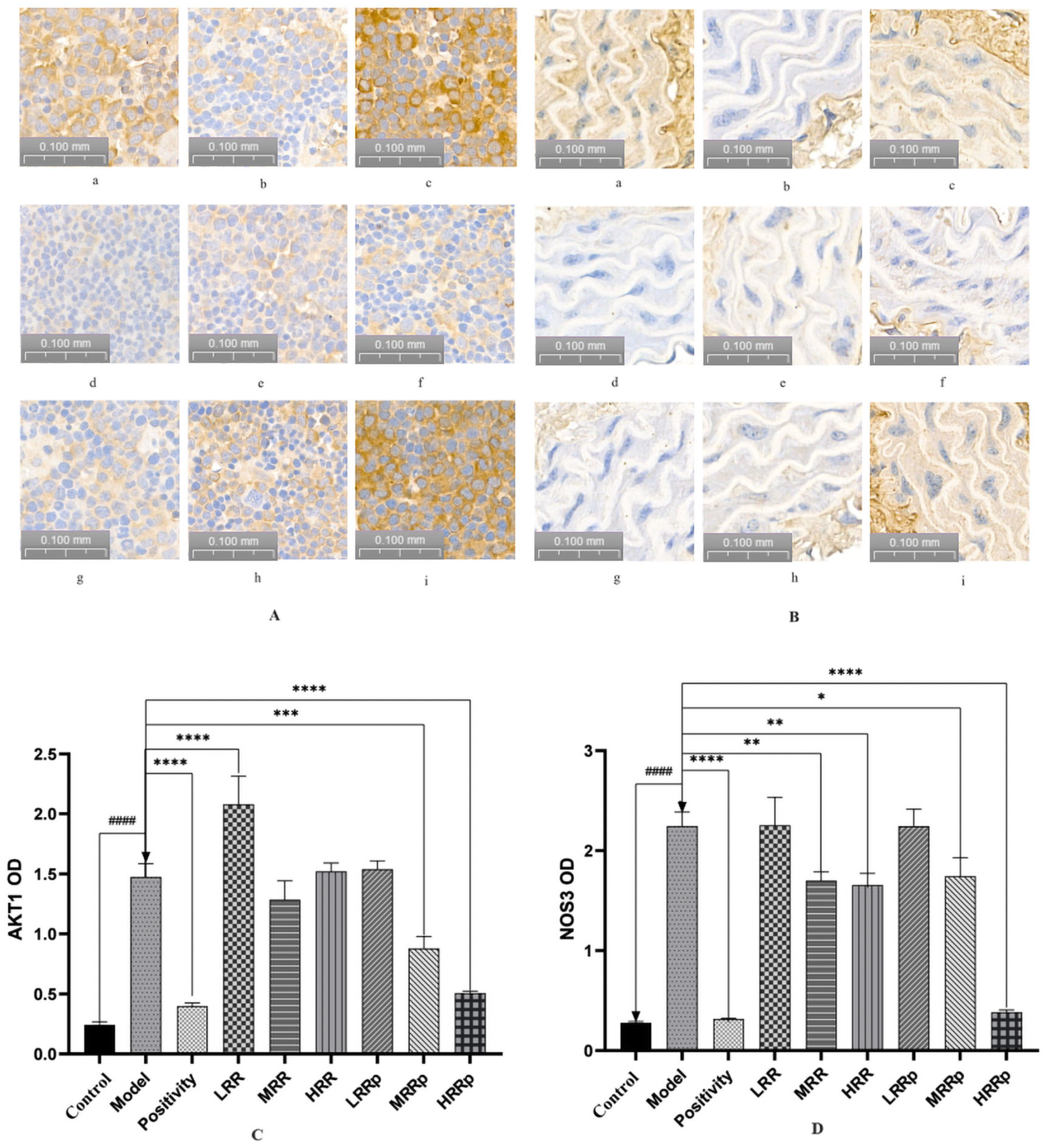
2.7. Results of Immunohistochemical Analysis of the Spleen and Abdominal Aorta
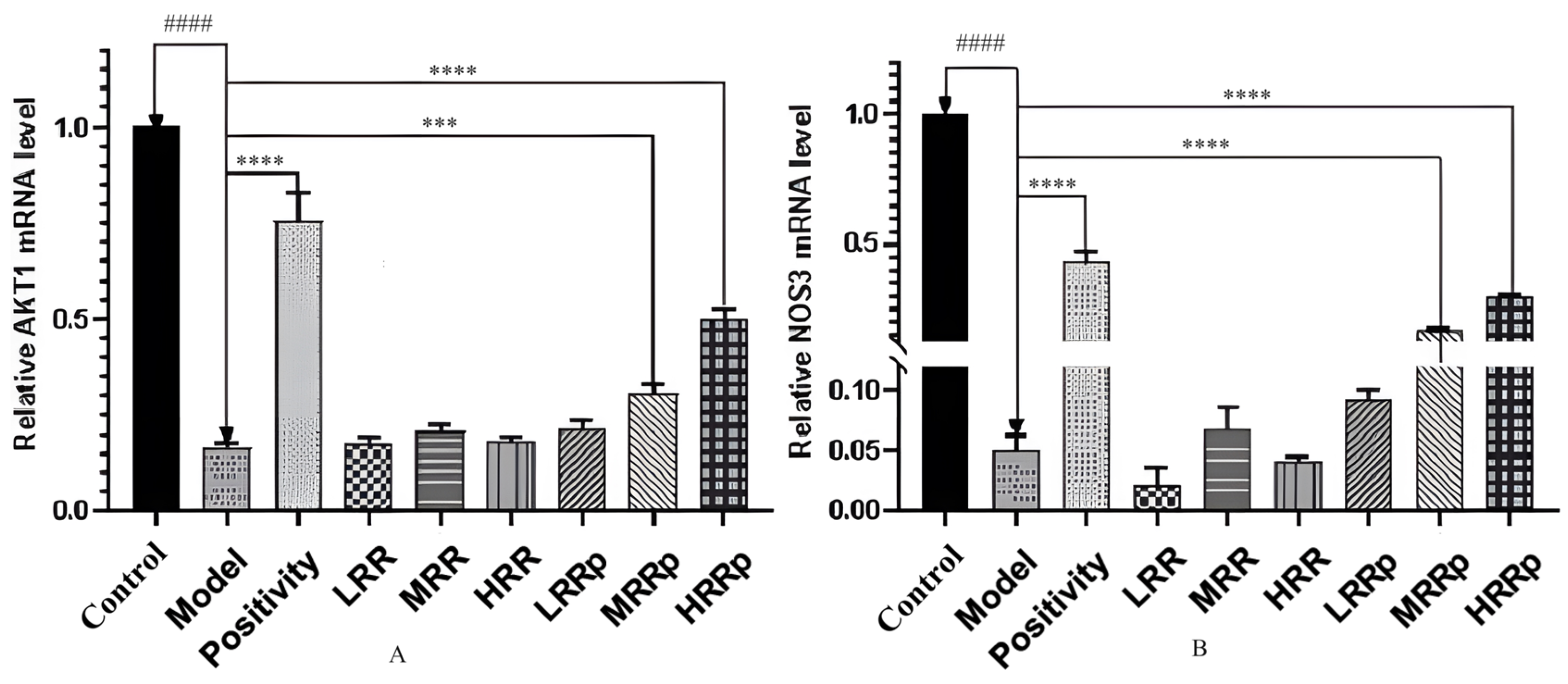
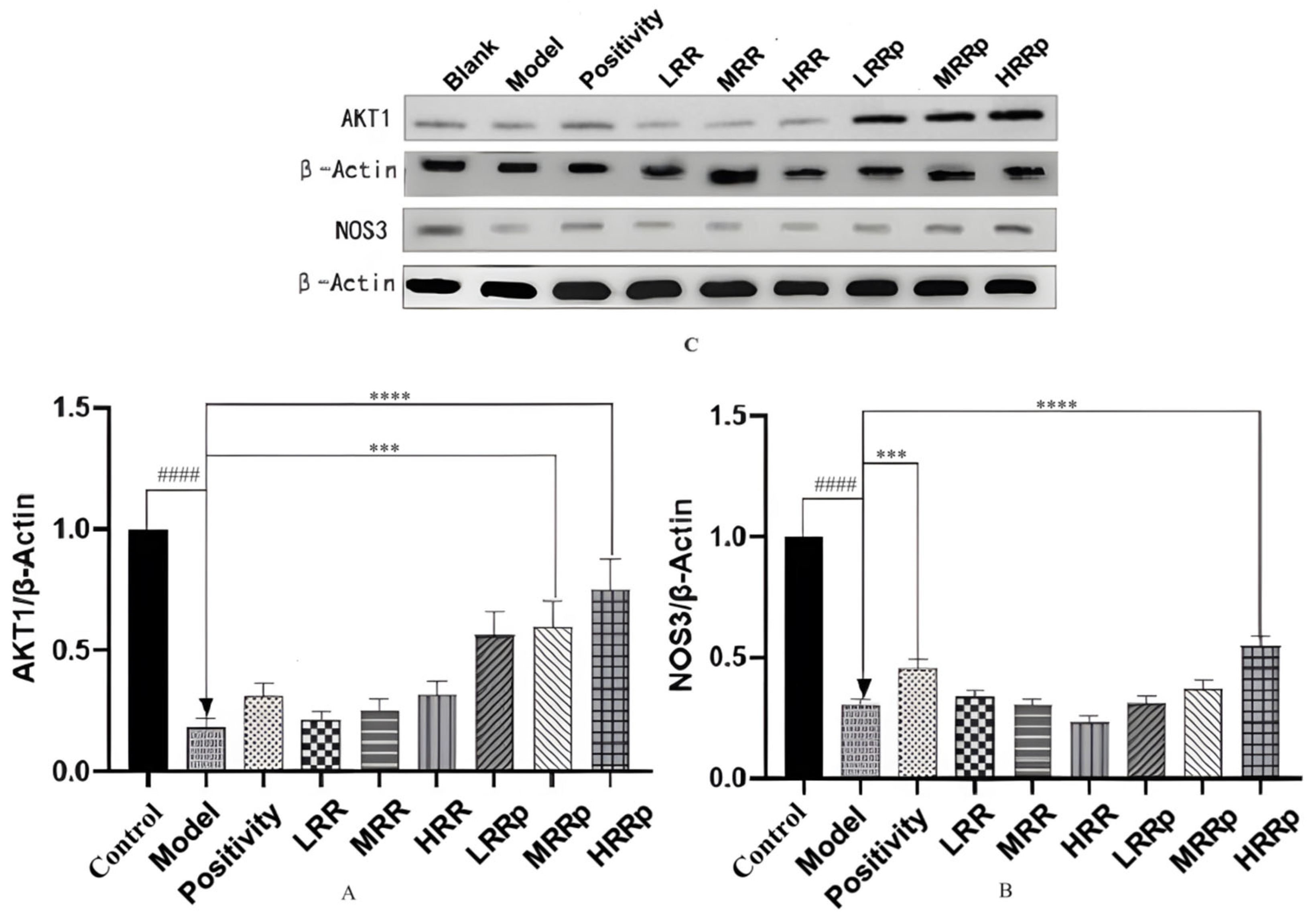
2.8. Expression of mRNAs in Tissues with AKT1 and NOS3
2.9. Expression of Proteins in Tissues with AKT1 and NOS3
3. Discussion
4. Materials and Methods
4.1. Instruments and Materials
4.2. Preparation of Test Solution
4.3. Detection Conditions
4.4. Mass Spectrometry Conditions
4.5. Network Analysis
4.6. Animals and Ethics Statement
4.7. Establishment of the Blood Deficiency Syndrome Model
4.8. Biochemical Index Detection of BDS Mice
4.8.1. Routine Blood Test
4.8.2. Determination of cAMP and cGMP Content in Plasma
4.8.3. Pathological Observation
4.8.4. Immunohistochemical Analysis
4.9. RT-qPCR Analysis
4.10. Western Blot Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| RP | Rehmanniae Radix |
| RRP | Rehmanniae Radix Praeparata |
| HGB | Hemoglobin |
| RBC | Red Blood Cells |
| PLT | Platelets |
| WBC | White Blood Cells |
| cAMP | Cyclic Adenosine Monophosphate |
| cGMP | Cyclic Guanosine Monophosphate |
| TCM | Traditional Chinese Medicine |
| BDS | Blood Deficiency Syndrome |
| HMDS | Hematopoietic–Metabolic Dysregulation Syndrome |
| LRR | Low-Dose Rehmanniae Radix Group |
| MRR | Middle-Dose Rehmanniae Radix Group |
| HRR | High-Dose Rehmanniae Radix Group |
| LRRP | Low-Dose Rehmanniae Radix Praeparata Group |
| MRRP | Middle-Dose Rehmanniae Radix Praeparata Group |
| HRRP | High-Dose Rehmanniae Radix Praeparata Group |
| APH | Acetylphenylhydrazine |
| CP | Cyclophosphamide |
| EDTA | Ethylenediaminetetraacetic Acid |
| ELISA | Enzyme-Linked Immunoassay |
| TCMSP | Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform |
| OMIM | Online Mendelian Inheritance in Man |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| GO | Gene Ontology |
| TBST | Tween 20 |
| BP | Biological Process |
| CC | Cell Composition |
| MF | Molecular Function |
| PCA | Principal Component Analysis |
| OPLS-DA | Orthogonal Partial Least Squares Discrimination Analysis |
References
- Wang, Y.; Li, C.; Chuo, W.; Liu, Z.; Ouyang, Y.; Li, D.; Han, J.; Wu, Y.; Guo, S.; Wang, W. Integrated proteomic and metabolomic analysis reveals the NADH-mediated TCA cycle and energy metabolism disorders based on a new model of chronic progressive heart failure. Mol. BioSyst. 2013, 9, 3135–3145. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Cui, N.; Su, F.; Wang, Y.; Yang, B.; Sun, Y.; Guan, W.; Kuang, H.; Wang, Q. Comprehensive Metabolomics and Network Pharmacology to Explore the Mechanism of 5-Hydroxymethyl Furfural in the Treatment of Blood Deficiency Syndrome. Front. Pharmacol. 2021, 12, 811331. [Google Scholar] [CrossRef]
- Li, X.; Dao, M.; Lykotrafitis, G.; Karniadakis, G.E. Biomechanics and biorheology of red blood cells in sickle cell anemia. J. Biomech. 2017, 50, 34–41. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Y.; Gao, M.; Cui, X.; Yang, Y.; van Duijn, B.; Wang, M.; Hu, Y.; Wang, C.; Xiong, Y. Steamed Panax notoginseng Attenuates Anemia in Mice with Blood Deficiency Syndrome via Regulating Hematopoietic Factors and JAK-STAT Pathway. Front. Pharmacol. 2019, 10, 1578. [Google Scholar] [CrossRef] [PubMed]
- Thu, V.K.; Thoa, N.T.; Hien, N.T.T.; Hang, D.T.T.; Kiem, P.V. Iridoid glycosides link with phenylpropanoids from Rehmannia glutinosa. Nat. Prod. Res. 2022, 36, 5370–5375. [Google Scholar] [CrossRef] [PubMed]
- Shu, P.; Yu, M.; Zhu, H.; Luo, Y.; Li, Y.; Li, N.; Zhang, H.; Zhang, J.; Liu, G.; Wei, X.; et al. Two new iridoid glycosides from Gardeniae Fructus. Carbohydr. Res. 2021, 501, 108259. [Google Scholar] [CrossRef]
- Xiao, Y.; Ren, Q.; Wu, L. The pharmacokinetic property and pharmacological activity of acteoside: A review. Biomed. Pharmacother. 2022, 153, 113296. [Google Scholar] [CrossRef]
- Ben Salem, K.; Ben Abdelaziz, A. Principal Component Analysis (PCA). Tunis. Med. 2021, 99, 383–389. [Google Scholar] [PubMed]
- Su, D.; Yang, Y.; Zeng, Q.; Liao, L.; Chen, C.; Yang, M.; Zhu, G.; Zhang, R.W.; Ai, Z.; Li, Y.; et al. Two Species Origins Comparison of Herba Patriniae Based on Their Ingredients Profile by UPLC-QTOF/MS/MS and Orthogonal Partial Least Squares Discriminant Analysis. Chem. Biodivers. 2022, 19, e202100961. [Google Scholar] [CrossRef]
- Bhattamisra, S.K.; Koh, H.M.; Lim, S.Y.; Choudhury, H.; Pandey, M. Molecular and Biochemical Pathways of Catalpol in Alleviating Diabetes Mellitus and Its Complications. Biomolecules 2021, 11, 323. [Google Scholar] [CrossRef]
- Bian, Z.; Zhang, R.; Zhang, X.; Zhang, J.; Xu, L.; Zhu, L.; Ma, Y.; Liu, Y. Extraction, structure and bioactivities of polysaccharides from Rehmannia glutinosa: A review. J. Ethnopharmacol. 2023, 305, 116132. [Google Scholar] [CrossRef] [PubMed]
- Safeukui, I.; Gomez, N.D.; Adelani, A.A.; Burte, F.; Afolabi, N.K.; Akondy, R.; Velazquez, P.; Holder, A.; Tewari, R.; Buffet, P.; et al. Malaria induces anemia through CD8+ T cell-dependent parasite clearance and erythrocyte removal in the spleen. mBio 2015, 6, e02493-14. [Google Scholar] [CrossRef]
- Altamura, S.; Vegi, N.M.; Hoppe, P.S.; Schroeder, T.; Aichler, M.; Walch, A.; Okreglicka, K.; Hultner, L.; Schneider, M.; Ladinig, C.; et al. Glutathione peroxidase 4 and vitamin E control reticulocyte maturation, stress erythropoiesis and iron homeostasis. Haematologica 2020, 105, 937–950. [Google Scholar] [CrossRef] [PubMed]
- Koldkjaer, P.; McDonald, M.D.; Prior, I.; Berenbrink, M. Pronounced in vivo hemoglobin polymerization in red blood cells of Gulf toadfish: A general role for hemoglobin aggregation in vertebrate hemoparasite defense? Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 305, R1190–R1199. [Google Scholar] [CrossRef] [PubMed]
- Caudrillier, A.; Kessenbrock, K.; Gilliss, B.M.; Nguyen, J.X.; Marques, M.B.; Monestier, M.; Toy, P.; Werb, Z.; Looney, M.R. Platelets induce neutrophil extracellular traps in transfusion-related acute lung injury. J. Clin. Investig. 2012, 122, 2661–2671. [Google Scholar] [CrossRef]
- Beauchamp, E.M.; Abedin, S.M.; Radecki, S.G.; Fischietti, M.; Arslan, A.D.; Blyth, G.T.; Yang, A.; Lantz, C.; Nelson, A.; Goo, Y.A.; et al. Identification and targeting of novel CDK9 complexes in acute myeloid leukemia. Blood 2019, 133, 1171–1185. [Google Scholar] [CrossRef]
- Zhang, Y.; Chandra, V.; Riquelme Sanchez, E.; Dutta, P.; Quesada, P.R.; Rakoski, A.; Zoltan, M.; Arora, N.; Baydogan, S.; Horne, W.; et al. Interleukin-17-induced neutrophil extracellular traps mediate resistance to checkpoint blockade in pancreatic cancer. J. Exp. Med. 2020, 217, e20190354. [Google Scholar] [CrossRef]
- Deng, Y.; Xie, M.; Li, Q.; Xu, X.; Ou, W.; Zhang, Y.; Xiao, H.; Yu, H.; Zheng, Y.; Liang, Y.; et al. Targeting Mitochondria-Inflammation Circuit by β-Hydroxybutyrate Mitigates HFpEF. Circ. Res. 2021, 128, 232–245. [Google Scholar] [CrossRef]
- Esai Selvan, M. Risk factors for death from COVID-19. Nat. Rev. Immunol. 2020, 20, 407. [Google Scholar] [CrossRef]
- Zhu, H.; Cao, C.; Wu, Z.; Zhang, H.; Sun, Z.; Wang, M.; Xu, H.; Zhao, Z.; Wang, Y.; Pei, G.; et al. The probiotic L. casei Zhang slows the progression of acute and chronic kidney disease. Cell Metab. 2021, 33, 1926–1942.e8. [Google Scholar] [CrossRef]
- Xiao, J.; Wang, S.; Chen, L.; Ding, X.; Dang, Y.; Han, M.; Zheng, Y.; Shen, H.; Wu, S.; Wang, M.; et al. 25-Hydroxycholesterol regulates lysosome AMP kinase activation and metabolic reprogramming to educate immunosuppressive macrophages. Immunity 2024, 57, 1087–1104.e7. [Google Scholar] [CrossRef] [PubMed]
- Hall, D.J.; Cui, J.; Bates, M.E.; Stout, B.A.; Koenderman, L.; Coffer, P.J.; Bertics, P.J. Transduction of a dominant-negative H-Ras into human eosinophils attenuates extracellular signal-regulated kinase activation and interleukin-5-mediated cell viability. Blood 2001, 98, 2014–2021. [Google Scholar] [CrossRef]
- Yao, Z.J.; Dong, J.; Che, Y.J.; Zhu, M.F.; Wen, M.; Wang, N.N.; Wang, S.; Lu, A.P.; Cao, D.S. TargetNet: A web service for predicting potential drug-target interaction profiling via multi-target SAR models. J. Comput. Aided Mol. Des. 2016, 30, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Tian, W.; Wang, Y.; Jin, X.; Guo, H.; Wang, Y.; Tang, Y.; Yao, X. Explore the mechanism and substance basis of Mahuang FuziXixin Decoction for the treatment of lung cancer based on network pharmacology and molecular docking. Comput. Biol. Med. 2022, 151, 106293. [Google Scholar] [CrossRef]
- Liu, W.; Fan, Y.; Tian, C.; Jin, Y.; Du, S.; Zeng, P.; Wang, A. Deciphering the Molecular Targets and Mechanisms of HGWD in the Treatment of Rheumatoid Arthritis via Network Pharmacology and Molecular Docking. Evid. Based Complement. Altern. Med. 2020, 2020, 7151634. [Google Scholar] [CrossRef]
- Pang, H.Q.; Yue, S.J.; Tang, Y.P.; Chen, Y.Y.; Tan, Y.J.; Cao, Y.J.; Shi, X.Q.; Zhou, G.S.; Kang, A.; Huang, S.L.; et al. Integrated Metabolomics and Network Pharmacology Approach to Explain Possible Action Mechanisms of Xin-Sheng-Hua Granule for Treating Anemia. Front. Pharmacol. 2018, 9, 165. [Google Scholar] [CrossRef]

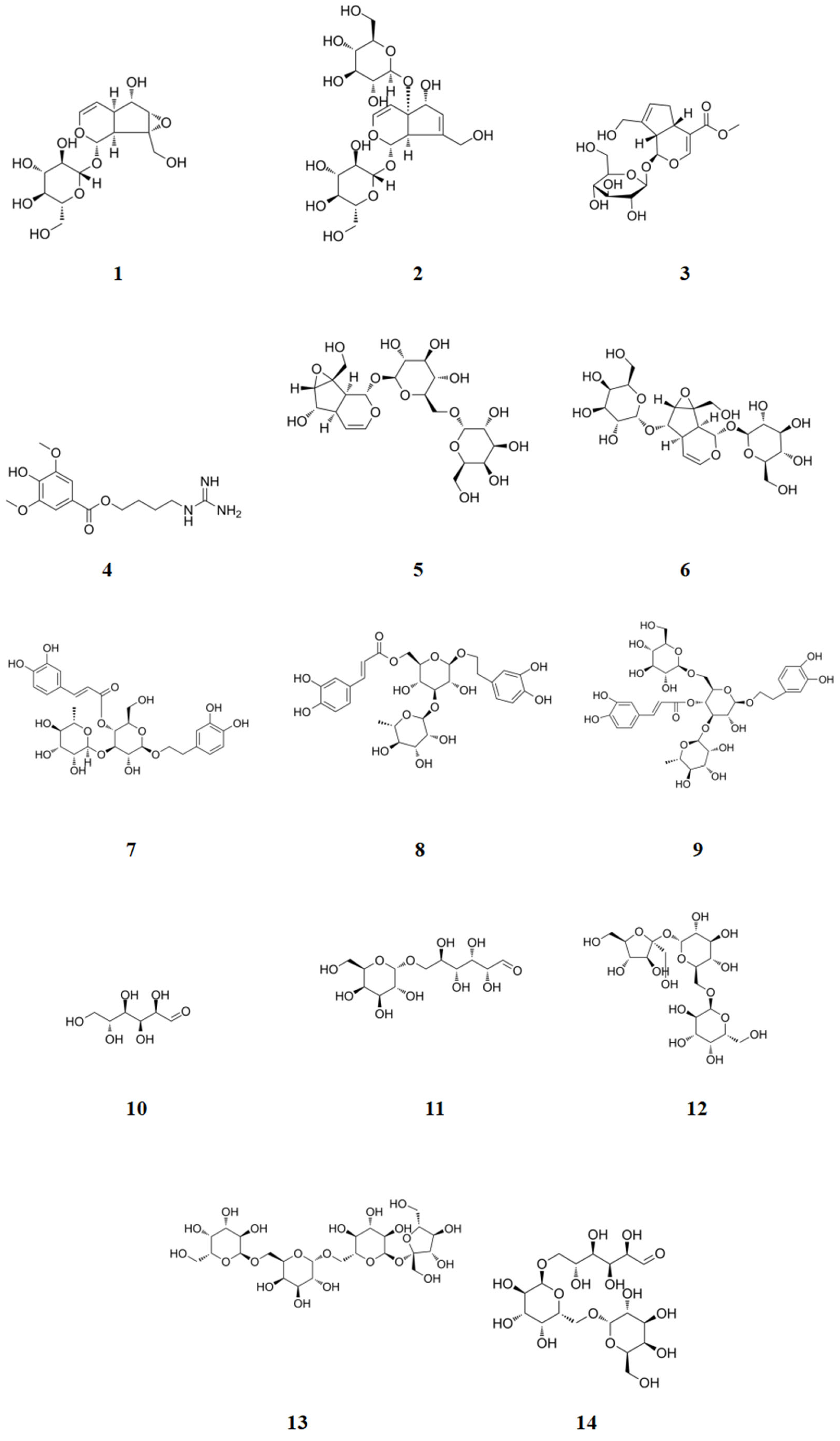



| Serial Number | Name of Compound | Chemical Formula | Molecular Weight |
|---|---|---|---|
| 1 | Catalpol | C15H22O10 | 362.33 |
| 2 | Melittoside | C21H32O15 | 524.47 |
| 3 | Geniposide | C17H24O10 | 388.37 |
| 4 | Leonuride | C15H24O9 | 348.35 |
| 5 | Rehmannioside A | C21H32O15 | 524.47 |
| 6 | Rehmannioside B | C21H32O15 | 524.47 |
| 7 | Verbascoside | C29H36O15 | 624.59 |
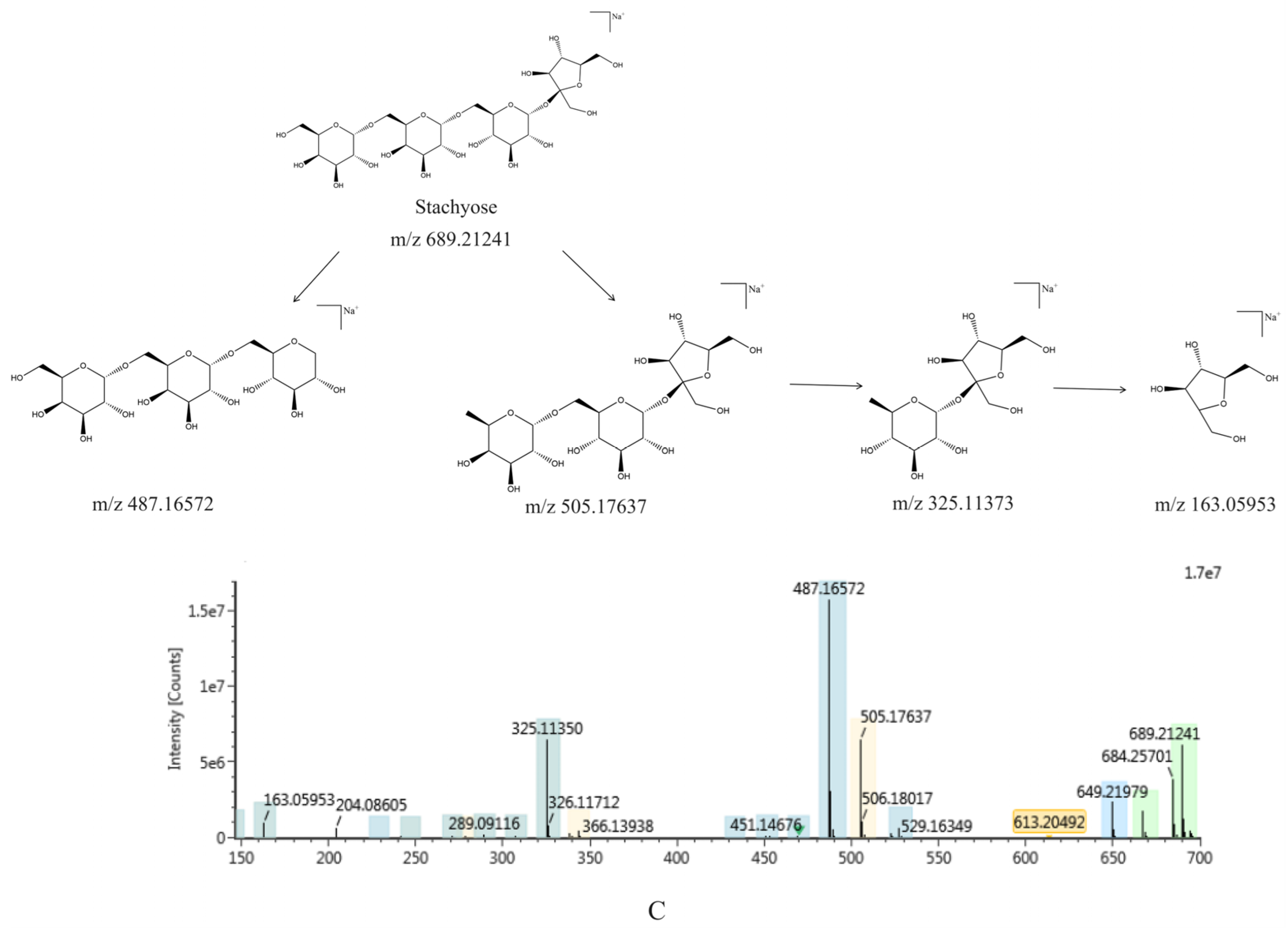
| 8 | Isoacteoside | C29H36O15 | 624.59 |
| 9 | Echinacoside | C35H46O20 | 786.73 |
| 10 | Glucose | C6H12O6 | 180.16 |
| 11 | Melibiose | C12H22O11 | 342.30 |
| 12 | Raffinose | C11H20O10 | 312.27 |
| 13 | Stachyose | C24H50O25 | 738.64 |
| 14 | Manninotriose | C18H32O16 | 504.44 |
| Component Name | Observed m/z | Mass Error (mDa) | Observed RT (min) | Adducts | Differentiating Ingredients | Content |
|---|---|---|---|---|---|---|
| Rehmaionoside B | 391.2326 | 0 | 1.27 | +H | Yes | + |
| Isoeugenol methyl ester | 179.1065 | −0.1 | 1.31 | +H | Yes | − |
| Dihydroxy-β-ionone | 249.1482 | 2 | 1.32 | +Na | No | |
| Eugenol | 165.0923 | 1.3 | 1.32 | +H | Yes | − |
| Stigmasterol | 413.3789 | 1.1 | 1.32 | +H | Yes | + |
| 1,5-bis(5-methoxymethyl)furan-2-yl-yenta-1,4-dien-3-one | 325.1058 | 1.1 | 1.38 | +Na | No | |
| Jinshengcaoflavin | 301.0731 | 2.5 | 1.57 | +H | No | |
| 5-hydroxymethyl furaldehyde | 127.0384 | −0.6 | 1.64 | +H | Yes | + |
| Rehmannia new terpenoid K | 712.3136 | −1.2 | 1.67 | +H | No | |
| 5-Hydroxymethylfurancarboxylic acid | 143.0333 | −0.6 | 1.85 | +H | Yes | − |
| Kojic acid | 269.1759 | 1.1 | 2.04 | +H | Yes | − |
| Nicotine | 163.1223 | −0.7 | 2.69 | +H | Yes | + |
| Isotachioside | 303.108 | 0.6 | 3.05 | +H | Yes | + |
| Masmin | 147.0903 | −1.4 | 3.14 | +H | No | |
| Sculponiside | 713.2817 | 3.7 | 3.69 | +Na | Yes | − |
| (2-phenylethyl-O-β-D-xylopyranosyl-(1→6)-β-D-glucopyranoside) | 413.2144 | −2.6 | 3.79 | +H | No | |
| Nornicotine | 149.107 | −0.3 | 4.32 | +H | No | |
| Rhodioloside | 323.1105 | 0.4 | 5.07 | +Na | No | |
| 1,2,5,6-tetrahydro-1-methyl-2-oxo-4-pyridine acetic acid | 170.0801 | −1 | 5.08 | +H | No | |
| Uracil | 113.0337 | −0.9 | 5.52 | +H | No | |
| 6-O-vanillate ajugol | 521.1672 | 4.3 | 5.53 | +Na | No | |
| Gardoside methyl ester | 389.144 | −0.2 | 5.56 | +H | Yes | + |
| Oxyrehmaionoside B | 429.2091 | −0.4 | 5.84 | +Na | Yes | − |
| Rehmaionoside C | 411.2002 | 1.3 | 5.98 | +Na | Yes | − |
| JionosideD | 639.2329 | 4.5 | 6.09 | +H | Yes | − |
| Nicotyrine | 181.0725 | −1.1 | 6.1 | +Na | Yes | + |
| Myobontioside A | 350.1202 | −0.6 | 6.12 | +H | Yes | + |
| 2,4-Dimethoxy-2-methyl-6H-pyran-3-one | 195.0638 | 1.1 | 6.38 | +Na | Yes | + |
| Trihydroxy-β-ionone | 265.1426 | 1.6 | 6.42 | +Na | No | |
| Isoquinoline | 130.0646 | −0.5 | 6.42 | +H | Yes | − |
| Rehmannia new terpenoid D | 431.2279 | 0.3 | 6.63 | +H | Yes | − |
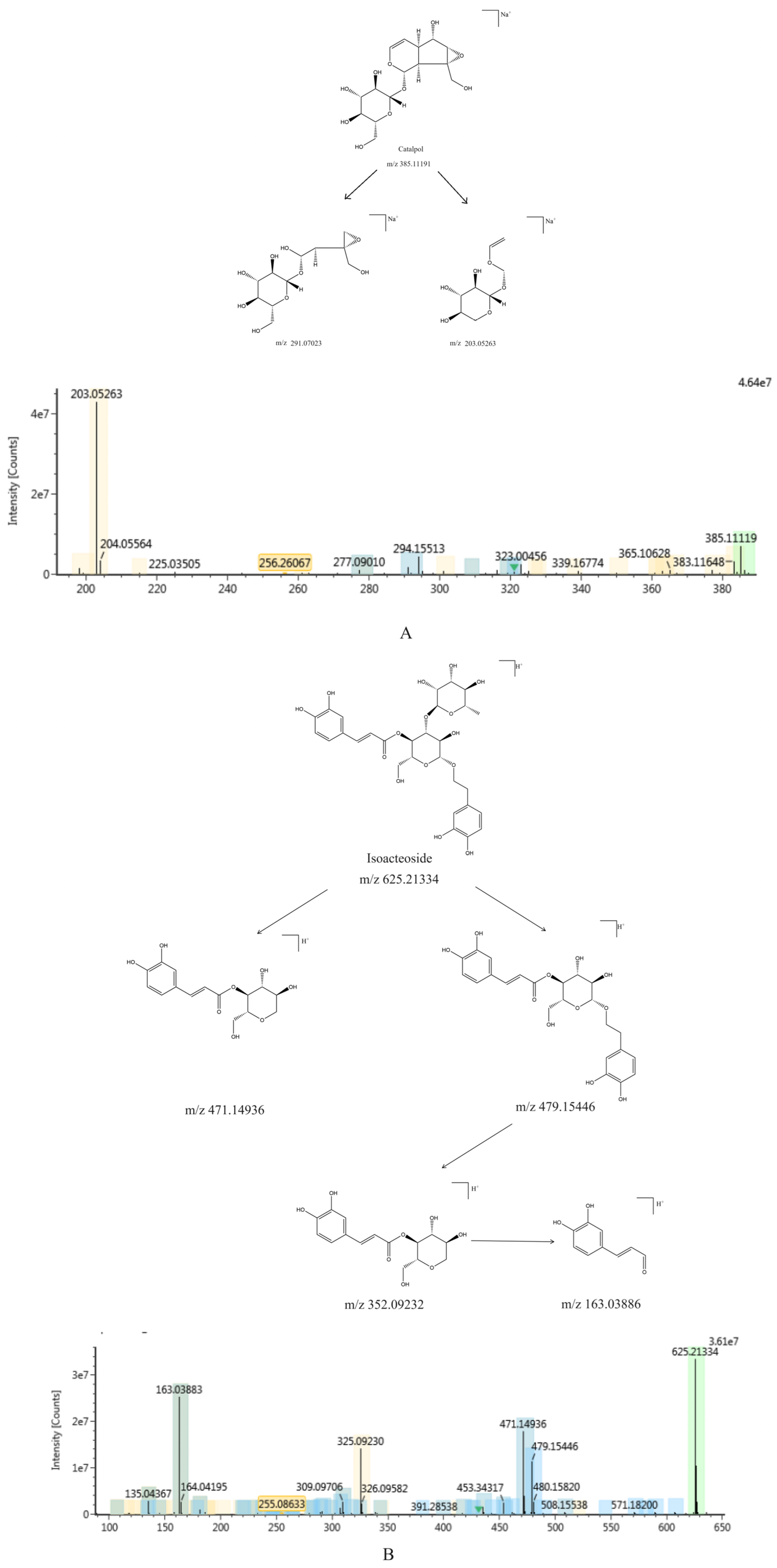
| Verbascoside | 625.2157 | 3 | 7.01 | +H | Yes | − |
| 6-O-sec-hydroxyaeginetoyl ajugol | 637.2837 | 0.7 | 7.08 | +Na | No | |
| (E)-4-(5-(hydroxymethyl)furan-2-yl)but-3-en-2-one | 189.0537 | 1.5 | 7.29 | +Na | No | |
| Mussaenosidic acid | 377.1479 | 3.6 | 7.3 | +H | Yes | − |
| Herniarin | 177.0553 | 0.7 | 7.36 | +H | No | |
| Lamiol | 401.1418 | −0.1 | 7.52 | +Na | Yes | + |
| Adenine | 136.0614 | −0.3 | 7.59 | +H | No | |
| Adenosine | 268.1046 | 0.5 | 7.6 | +H | No | |
| 5-Deoxylamiol | 363.1619 | −3.1 | 7.85 | +H | Yes | − |
| Myoporoside | 349.1492 | −0.1 | 7.86 | +H | Yes | + |
| Genipin 1-O-a-L-rhamnopyranosyl(1→6)-β-D-glucopyranoside | 535.2028 | 0.6 | 7.89 | +H | Yes | + |
| Hydrocoumarin | 149.0594 | −0.3 | 8.01 | +H | No | |
| Jionoside B1 | 815.3007 | 3.8 | 8.16 | +H | Yes | − |
| Uridine | 245.076 | −0.8 | 8.17 | +H | No | |
| Martynoside | 653.2462 | 2.2 | 8.18 | +H | No | − |
| Galactose | 203.053 | 0.4 | 9.12 | +Na | No | |
| Jionoside A1 | 801.2876 | 6.5 | 9.2 | +H | Yes | + |
| Cis-asarone | 231.1008 | 1.7 | 9.38 | +Na | No | |
| Dihydrocatalpol | 387.1273 | 1.1 | 9.4 | +Na | Yes | − |
| Ethylvanillin | 167.0712 | 0.9 | 9.4 | +H | Yes | − |
| Ferulic acid | 195.0662 | 1 | 9.61 | +H | Yes | + |
| Catalpinoside | 385.1111 | 0.6 | 10.09 | +Na | Yes | − |
| Coumarin | 147.0436 | −0.5 | 12.03 | +H | Yes | − |
| Benzoic acid | 123.0442 | 0.2 | 12.04 | +H | Yes | + |
| Rehmannioside C | 533.1837 | −0.3 | 12.17 | +Na | No | |
| Dibutylphthalate | 301.1419 | 0.8 | 12.31 | +Na | Yes | + |
| Syringic acid | 199.0596 | −0.5 | 12.51 | +H | Yes | − |
| Feruloyl ajugol | 743.2507 | −1.4 | 13.29 | +Na | No | |
| 7-methylcoumarin | 161.0605 | 0.8 | 13.81 | +H | Yes | − |
| Rehmannia new terpenoid J | 696.2809 | −2.6 | 13.83 | +H | Yes | + |
| (7R,8S,7′R,8′,S′)-4,9,4′,9′-tetrahydroxy-3,3′-dimethoxy-7,7′-epoxylignan 9-O-β-D-glucopyranoside | 539.2115 | −0.8 | 14.15 | +H | Yes | + |
| Dhydrophaseic acid 4′-O-β-D-glucopyranoside | 467.1887 | −0.1 | 14.3 | +Na | Yes | + |
| 6-O-E-caffeoyl ajugol | 533.1609 | −2.1 | 14.44 | +Na | No | |
| Rhinanthin | 347.1345 | 0.8 | 14.72 | +H | Yes | − |
| Manninotriose | 527.161 | 2.7 | 14.77 | +Na | No | |
| Retinoid D | 709.2195 | 3.4 | 14.81 | +Na | No | |
| Sucrose | 365.1065 | 1.1 | 15.02 | +Na, +H | No | |
| Sec-hydroxyaeginetic acid | 307.153 | 1.4 | 15.26 | +Na | No | |
| Catapolgenin | 201.0757 | 0 | 15.27 | +H | No | |
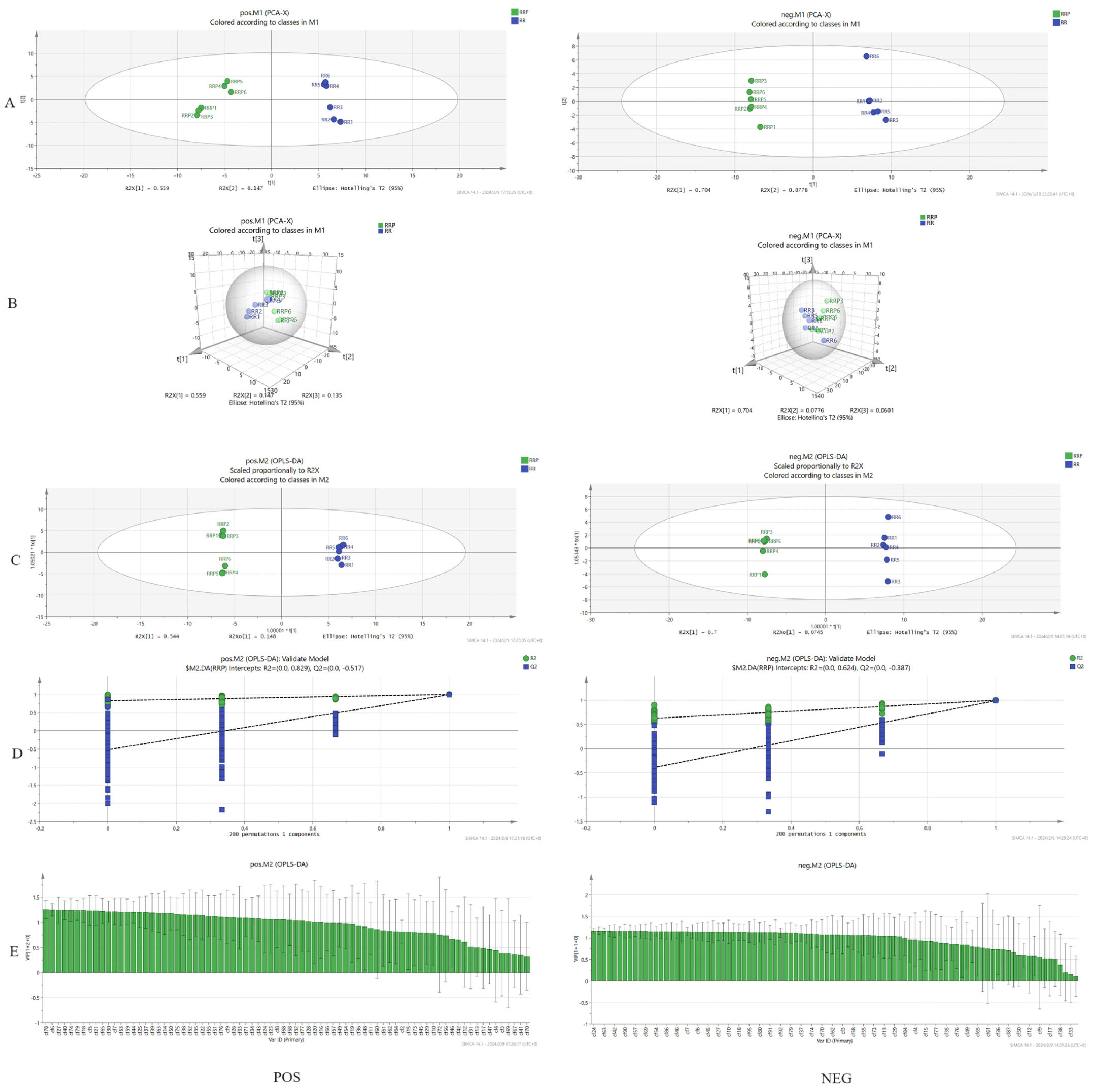
| Stachyose | 667.2305 | 1.3 | 15.52 | +H | Yes | − |
| Melittin monoglucoside | 363.1289 | 0.3 | 16.03 | +H | Yes | − |
| Rehmannia new terpenoid B | 323.1461 | −0.4 | 16.3 | +Na | No | |
| Rehmannia neoterpene E | 447.2224 | −0.1 | 16.8 | +H | Yes | − |
| Raffinose | 505.1765 | 0.2 | 17.03 | +H, +Na | No | |
| Rehmannioside A | 525.1795 | −1.8 | 18.05 | +H | No | |
| Rehmannia new glucoside A | 495.2258 | 3.3 | 18.08 | +H | Yes | + |
| yemuoside YM11 | 507.1903 | 4.3 | 18.86 | +H | Yes | + |
| 7-ethoxy-4-methylcoumarin | 227.0663 | −1.5 | 19.82 | +Na | Yes | − |
| Component Name | Observed m/z | Mass Error (mDa) | Observed RT (min) | Adducts | Differentiating Ingredients | Content |
|---|---|---|---|---|---|---|
| Myobontioside A | 433.2053 | −2.6 | 1.27 | +HCOO | Yes | − |
| vanillin | 457.2123 | 4.3 | 1.4 | +HCOO | Yes | + |
| Rehmannia new glucoside B | 211.1332 | −0.8 | 1.42 | -H, +HCOO | Yes | + |
| verbascoside | 455.3524 | −0.6 | 1.42 | -H | Yes | − |
| Dihydroxy-β-ionone | 225.1483 | −1.4 | 1.46 | -H, +HCOO | No | |
| 5-hydroxymethyl-2-furancarboxaldehyde | 429.2487 | −0.7 | 1.47 | -H | Yes | + |
| p-methoxycinnamic acid | 223.0983 | 0.7 | 1.5 | +HCOO, -H | Yes | + |
| yemuoside YM1 | 209.0836 | 1.7 | 1.51 | +HCOO | No | |
| Rehmapicrogenin | 183.1013 | −1.4 | 1.51 | -H | No | |
| 1,5-bis(5-methoxymethyl)furan-2-yl-yenta-1,4-dien-3-one | 347.1164 | 2.8 | 1.54 | +HCOO, -H | Yes | − |
| (2-phenylethyl-O-β-D-xylopyranosyl-(1→6)-β-D-glucopyranoside) | 205.0498 | −0.8 | 1.54 | +HCOO | Yes | − |
| 7-ethoxy-4-methylcoumarin | 249.0777 | 0.9 | 1.55 | +HCOO | Yes | + |
| sucrose | 193.0498 | −0.8 | 1.56 | -H, +HCOO | Yes | − |
| ajugol | 621.4376 | 0.4 | 1.57 | +HCOO | Yes | − |
| eugenol | 225.0768 | 0 | 1.59 | -H | No | |
| Rehmannioside C | 223.0606 | −0.6 | 1.6 | +HCOO, -H | Yes | − |
| ethylvanillin | 165.0544 | −1.3 | 1.61 | -H, +HCOO | Yes | + |
| herniarin | 221.0451 | −0.5 | 1.62 | +HCOO | Yes | + |
| ferulic acid | 151.0388 | −1.2 | 1.64 | -H, +HCOO | Yes | + |
| sec-hydroxyaeginetic acid | 283.1535 | −1.6 | 1.64 | -H | Yes | − |
| Genipin-gentiobioside | 213.075 | −1.8 | 1.67 | +HCOO | Yes | − |
| rhinanthin | 199.0601 | −1.1 | 2.07 | -H | Yes | + |
| succinic acid | 197.0444 | −1.1 | 2.55 | -H | No | |
| 5-Hydroxymethylfurancarboxylic acid | 187.024 | −0.8 | 2.73 | +HCOO | No | |
| Trihydroxy-β-ionone | 287.1517 | 1.7 | 2.96 | +HCOO | No | |
| Isoscrophularigenin | 215.0918 | −0.7 | 3.13 | +HCOO | Yes | + |
| 1,2,5,6-tetrahydro-1-methyl-2-oxo-4-pyridineacetic acid | 214.0711 | −1 | 3.15 | +HCOO | No | |
| benzoic acid | 167.0341 | −0.9 | 3.16 | +HCOO | Yes | + |
| 3-methoxy-4-hydroxybenzoic acid | 213.0395 | −0.9 | 3.21 | +HCOO, -H | Yes | + |
| Rehmaionoside B | 301.0928 | −0.1 | 3.28 | -H | Yes | − |
| Rehmannia new terpenoid D | 429.2115 | −1.5 | 4.25 | -H, +HCOO | No | |
| Oxyrehmaionoside B | 435.2274 | 3.8 | 4.3 | +HCOO, -H | Yes | − |
| Rehmannia new glucosideJ | 345.1552 | −0.2 | 4.32 | +HCOO | Yes | + |
| Melittin | 697.2363 | 1.4 | 4.47 | +HCOO, -H | Yes | + |
| 6-O-E-feruloyl ajugol | 569.1908 | 3.2 | 4.59 | +HCOO, -H | Yes | − |
| 8-epiloganic acid | 375.1293 | −0.4 | 4.63 | -H | No | |
| Geniposide | 345.1182 | −0.9 | 4.68 | -H | No | |
| 6-O-vanillate ajugol | 543.1729 | 1 | 4.72 | +HCOO, -H | Yes | − |
| Tachioside | 163.0237 | −1.1 | 4.77 | +HCOO | No | |
| Cis-rehmannioside | 449.2035 | 0.6 | 4.81 | +HCOO | Yes | − |
| geniposide | 433.1354 | 0.2 | 4.81 | +HCOO | Yes | + |
| Choulongjianoside A | 433.1355 | 0.4 | 4.82 | +HCOO | Yes | − |
| JionosideD | 637.2146 | 0.8 | 4.96 | -H | No | |
| Angeloside C | 243.0613 | −1 | 4.96 | -H, +HCOO | Yes | + |
| (7R,8S,7′R,8′,S′)-4,9,4′,9′-tetrahydroxy-3,3′-dimethoxy-7,7′-epoxylignan 9-O-β-D-glucopyranoside | 583.2048 | 1.5 | 5.01 | +HCOO | Yes | − |
| nonen-1-one | 451.2189 | 0.4 | 5.02 | +HCOO | Yes | + |
| Rehmannia new terpenoid A | 445.2082 | 0.3 | 5.05 | -H, +HCOO | No | |
| neo-rehmannioside | 477.2351 | 0.9 | 5.1 | +HCOO | Yes | − |
| Jioglutoside B | 563.1998 | 1.7 | 5.13 | +HCOO | No | |
| feruloyl ajugol | 719.2571 | 1.5 | 5.14 | -H | No | |
| 2,4-Dimethoxy-2-methyl-6H-pyran-3-one | 217.0708 | −1 | 5.16 | +HCOO | Yes | − |
| Rehmannia new terpenoid F | 659.326 | −2.4 | 5.19 | +HCOO, -H | Yes | − |
| 5-deoxyantirrhinoside | 391.1251 | 0.5 | 5.21 | +HCOO | Yes | + |
| Dhydrophaseic acid 4′-O-β-D-glucopyranoside | 443.192 | −0.2 | 5.29 | -H | Yes | − |
| raffinose | 623.1991 | 1 | 5.42 | -H | Yes | − |
| Melittin monoglucoside | 461.1667 | 0.3 | 5.62 | -H | No | |
| Isoeugenol methyl ester | 373.1132 | −0.8 | 5.8 | -H, +HCOO | No | |
| gardoside methyl ester | 373.1132 | −0.8 | 5.8 | -H, +HCOO | No | |
| Rehmannioside A | 569.1737 | 1.4 | 5.82 | +HCOO | Yes | + |
| 5-Deoxylamiol | 407.1569 | 1.1 | 5.92 | +HCOO | Yes | − |
| Jionoside B1 | 813.2835 | 1.2 | 5.95 | -H, +HCOO | No | |
| Genipin 1-O-a-L-rhamnopyranosyl(1→6)-β-D-glucopyranoside | 579.1984 | 5.3 | 5.96 | +HCOO | No | |
| Carrot glycosides A | 179.055 | −1.1 | 6.01 | -H | No | |
| 1-methyl-1,2,3,4-tetrahydro-β-carboline-3-carboxylic acid | 291.1358 | 0.8 | 6.04 | +HCOO | No | |
| Cistanoside F | 312.0945 | −0.5 | 6.08 | +HCOO | Yes | + |
| Decaffeoyl verbascoside | 721.2519 | −4.2 | 6.12 | +HCOO | Yes | − |
| ursolic acid | 393.1396 | −0.6 | 6.22 | +HCOO | NO | |
| Rehmannia new terpenoid J | 740.2719 | −2.6 | 6.32 | +HCOO | Yes | + |
| manninotriose | 487.1459 | 0.2 | 6.37 | -H | Yes | − |
| Jionoside A1 | 799.2674 | 0.8 | 6.42 | -H, +HCOO | No | |
| lamiol | 409.1349 | −0.3 | 6.87 | +HCOO, -H | No | |
| Rehmanniae neoterpene C | 551.2823 | −3.8 | 6.89 | -H | No | |
| syringic acid | 407.1192 | −0.3 | 7.13 | +HCOO | No | |
| Genameside C | 394.1133 | 1.6 | 7.13 | +HCOO | Yes | + |
| catalpinoside | 407.1192 | −0.3 | 7.14 | +HCOO | Yes | − |
| genipin | 423.1544 | 3.6 | 7.81 | +HCOO | No | |
| 7-methylcoumarin | 595.187 | −0.9 | 7.85 | +HCOO | No | |
| Rehmannia new terpenoid B | 509.1882 | 0.7 | 7.9 | -H | Yes | + |
| 6-O-α-D-galactosyl harpagide | 655.2293 | 4.9 | 8.5 | -H, +HCOO | No | |
| glucose | 569.1755 | 3.2 | 8.77 | +HCOO, -H | Yes | + |
| Rehmannia neoterpene E | 829.2788 | 1.6 | 8.84 | +HCOO | Yes | − |
| adenosine | 387.1137 | −0.7 | 9.06 | +HCOO, -H | Yes | − |
| Mussaenosidic acid | 421.1359 | 0.8 | 9.27 | +HCOO | Yes | + |
| Masitic acid | 549.1678 | 0.6 | 9.59 | +HCOO, -H | Yes | − |
| Rehmannioside D | 731.227 | 1.9 | 9.72 | +HCOO | Yes | + |
| melasmoside | 125.0237 | −0.7 | 9.98 | -H | Yes | − |
| Rehmaionoside C | 551.1781 | 1.1 | 10.26 | +HCOO, -H | No | |
| stachyose | 503.1614 | −0.4 | 10.55 | -H | Yes | − |
| verbascose | 711.2204 | 0.3 | 11.1 | +HCOO, -H | Yes | − |
| harpagoside | 740.277 | 2.6 | 11.41 | +HCOO | No | |
| Leucosceptoside A | 637.2134 | −0.4 | 11.43 | -H | Yes | + |
| Sculponiside | 735.2906 | 3.6 | 11.63 | +HCOO | Yes | − |
| Aeginetic acid 5-O-β-D-quinovoside | 595.1868 | −1.2 | 11.85 | +HCOO | No | |
| catapolgenin | 873.2743 | 1.5 | 12.08 | +HCOO, -H | No | |
| uridine | 517.1576 | 1.3 | 13.18 | +HCOO | No |
| Active Ingredient Target | Binding Energy (kcal/mol) | |
|---|---|---|
| AKT1 | NOS3 | |
| D-Fructose | −4.5 | −5.9 |
| Glucose | −4.8 | −5.5 |
| 5-hydroxymethylfurfural | −4.3 | −5.3 |
| Melibiose | −6 | −7.5 |
| Citric Acid | −4.6 | −5.3 |
| 8-Epilganic Acid | −6.9 | −7.5 |
| Manninotriose | −6.1 | −6.5 |
| Primers | Forward | Reverse |
|---|---|---|
| AKT1 | AGAGGCAGGAAGAAGAGACGATGG | GCAGGACACGGTTCTCAGTAAGC |
| NOS3 | GCAGGCATCACCAGGAAGAAGAC | CTGAGCAGGAGACACTGTTGAATCG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, R.; Wang, F.; Liu, X.; Yuan, C.; Shan, G. Rehmanniae Radix Praeparata in Blood Deficiency Syndrome: UPLC-Q-TOF-MS Profiling, Network Pharmacology, and PI3K-AKT Activation. Int. J. Mol. Sci. 2025, 26, 3914. https://doi.org/10.3390/ijms26083914
Gao R, Wang F, Liu X, Yuan C, Shan G. Rehmanniae Radix Praeparata in Blood Deficiency Syndrome: UPLC-Q-TOF-MS Profiling, Network Pharmacology, and PI3K-AKT Activation. International Journal of Molecular Sciences. 2025; 26(8):3914. https://doi.org/10.3390/ijms26083914
Chicago/Turabian StyleGao, Ruxi, Fanyi Wang, Xiang Liu, Chu Yuan, and Guoshun Shan. 2025. "Rehmanniae Radix Praeparata in Blood Deficiency Syndrome: UPLC-Q-TOF-MS Profiling, Network Pharmacology, and PI3K-AKT Activation" International Journal of Molecular Sciences 26, no. 8: 3914. https://doi.org/10.3390/ijms26083914
APA StyleGao, R., Wang, F., Liu, X., Yuan, C., & Shan, G. (2025). Rehmanniae Radix Praeparata in Blood Deficiency Syndrome: UPLC-Q-TOF-MS Profiling, Network Pharmacology, and PI3K-AKT Activation. International Journal of Molecular Sciences, 26(8), 3914. https://doi.org/10.3390/ijms26083914






