Antioxidant and Neuroprotective Properties of Selected Pyrrole-Containing Azomethine Compounds in Neurotoxicity Models In Vitro
Abstract
1. Introduction
2. Results
2.1. Selection of the Model Compounds
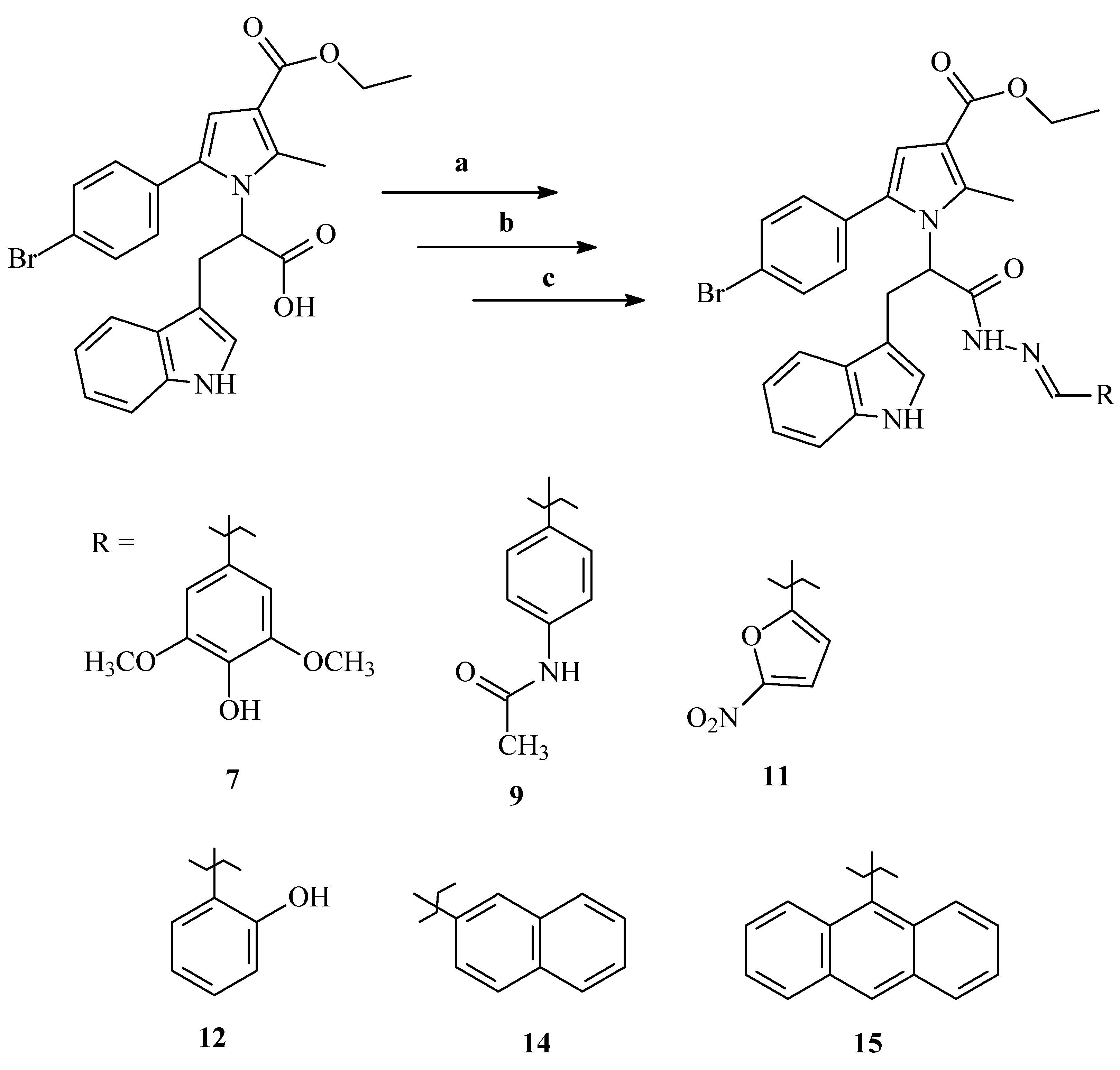
2.2. Synthesis of the Selected Molecules
2.3. In Silico Characterization of the Physicochemical Properties of the Target Compounds
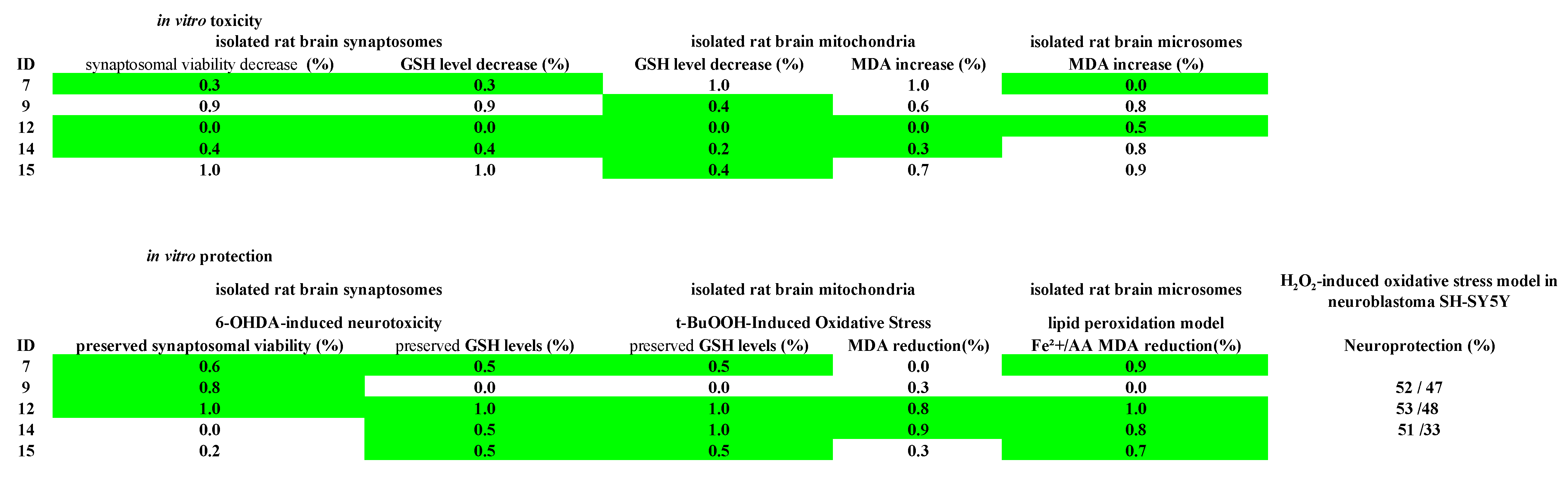
2.4. In Vitro Toxicity Evaluation of the Target Pyrrole Hydrazones on Subcellular and Cellular Models
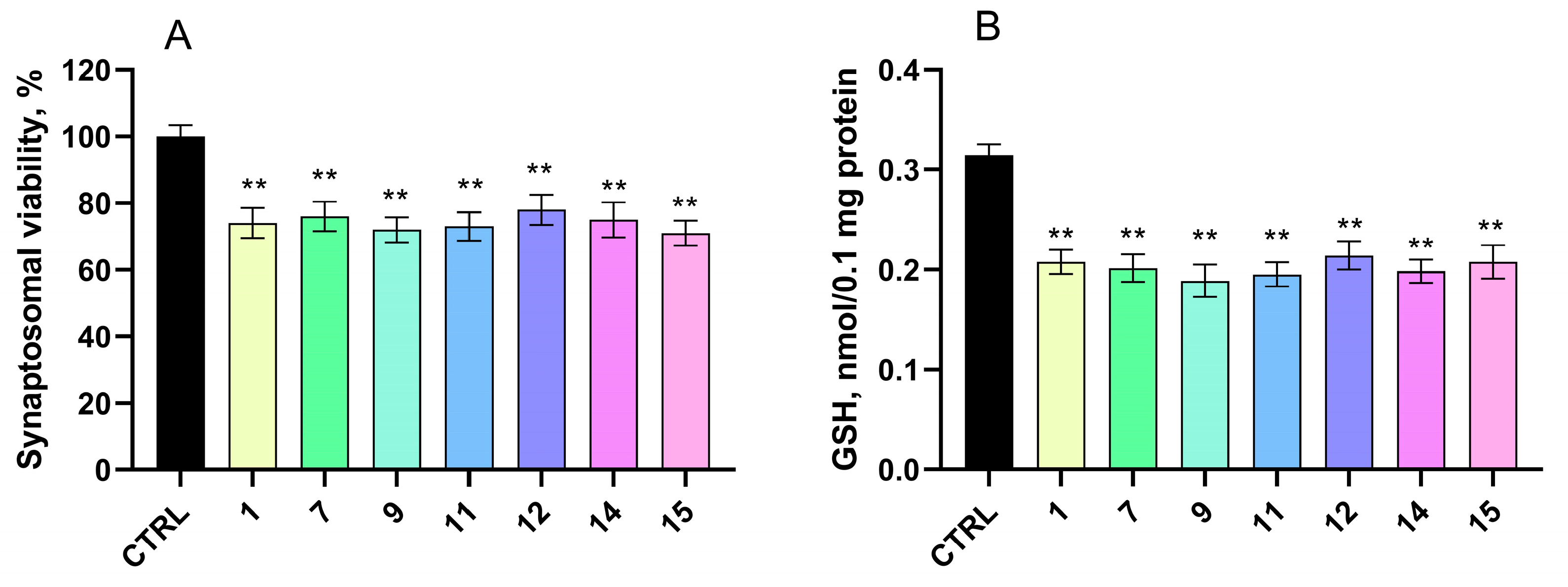
2.4.1. Effects on Isolated Rat Brain Synaptosomes
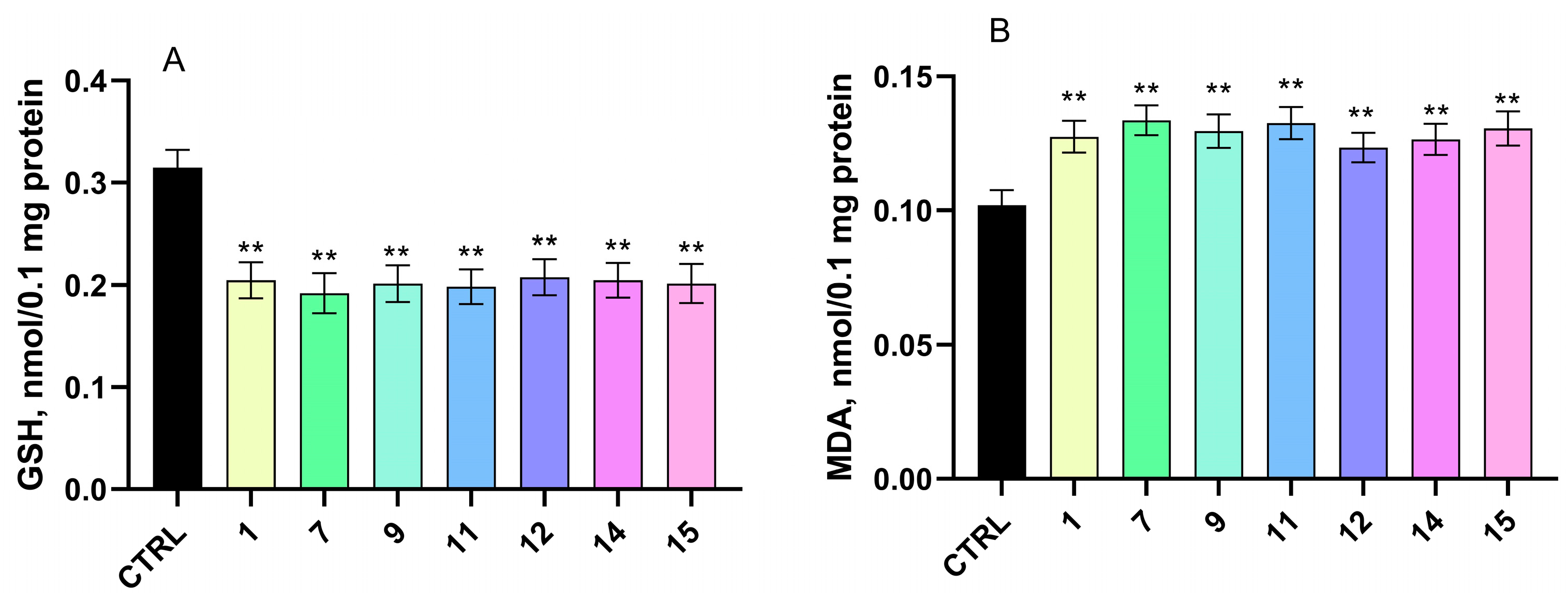
2.4.2. Effects on Isolated Rat Brain Mitochondria
2.4.3. Effects on Isolated Rat Brain Microsomes
2.4.4. In Vitro Cytotoxicity Evaluation in Neuroblastoma Cell Line SH-SY5Y
2.5. Protective Effects of the Target Compounds in In Vitro Models
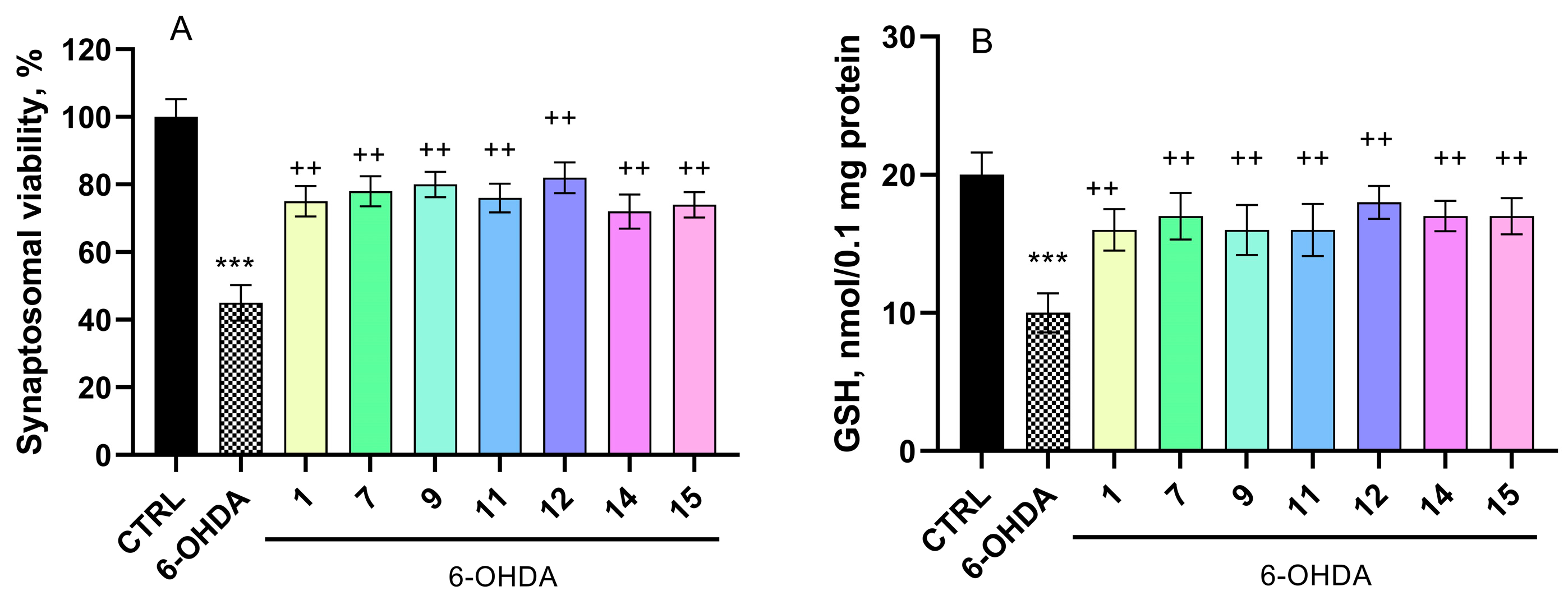
2.5.1. Effects on a Model of 6-OHDA-Induced Neurotoxicity in Isolated Rat Brain Synaptosomes
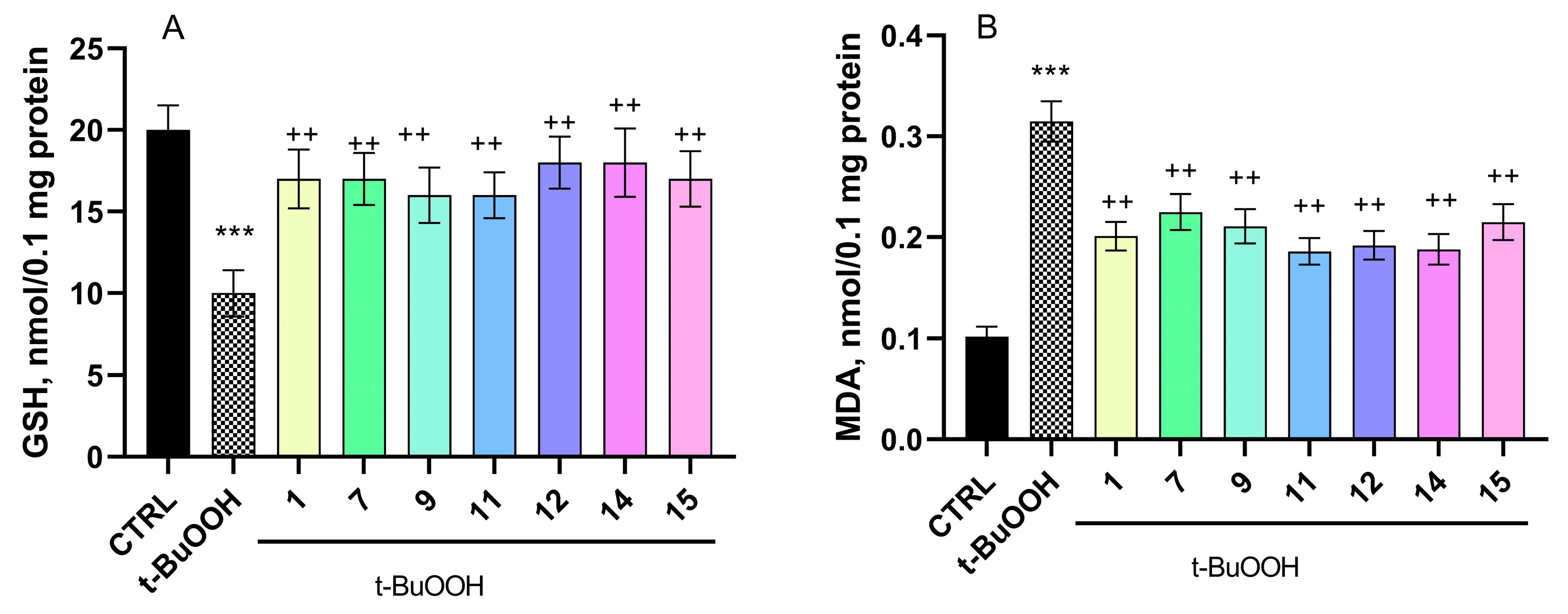
2.5.2. Effect of Pyrrole Hydrazones on t-BuOOH-Induced Oxidative Stress in Brain Mitochondria
2.5.3. Effect of Pyrrole Hydrazones in a Model of Non-Enzyme Lipid Peroxidation in Isolated Rat Brain Microsomes
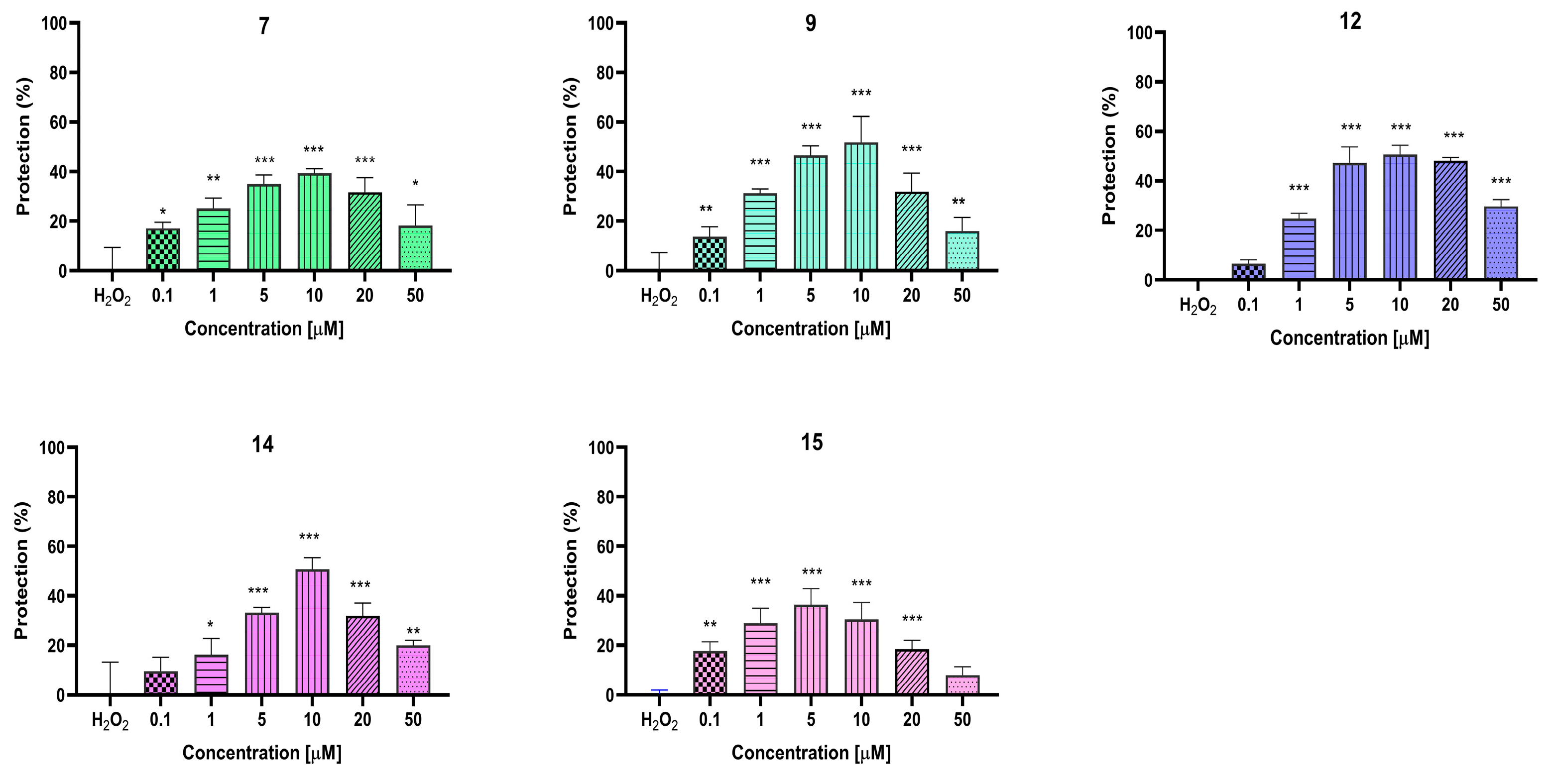
2.6. In Vitro Evaluation of the Neuroprotective Effects of the Target Pyrrole Hydrazones on Neuroblastoma SH-SY5Y Cells
3. Discussion
4. Materials and Methods
4.1. Chemistry
4.2. In Silico Calculation of Physicochemical Properties
4.3. Experimental Animals
4.4. Preparation of Rat Brain Synaptosomes and Mitochondria
4.5. Isolation of Synaptosomes and Mitochondria
4.6. 6-OHDA-Induced Neurotoxicity
4.7. MTT Assay to Assess the Synaptosomal Viability
4.8. Determination of Reduced Glutathione (GSH) in Isolated Brain Synaptosomes
4.9. Tert-Butyl Hydroperoxide-Induced Oxidative Stress
4.10. Determination of Malondialdehyde (MDA) Production in Brain Mitochondria
4.11. Determination of the GSH Level in Brain Mitochondria
4.12. Isolation of Brain Microsomes
4.13. Iron-/Ascorbate-Induced Lipid Peroxidation (LPO)
4.14. Determination of MDA in Brain Microsomes
4.15. Cell Cultures and Treatment
4.16. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Dias, V.; Junn, E.; Mouradian, M.M. The Role of Oxidative Stress in Parkinson’s Disease. J. Park. Dis. 2013, 3, 461–491. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liu, Y.; Zhou, J. Neuroinflammation in Parkinson’s Disease and Its Potential as Therapeutic Target. Transl. Neurodegener. 2015, 4, 19. [Google Scholar] [CrossRef]
- Hussain, H.; Ahmad, S.; Shah, S.W.A.; Ullah, A.; Rahman, S.U.; Ahmad, M.; Almehmadi, M.; Abdulaziz, O.; Allahyani, M.; Alsaiari, A.A.; et al. Synthetic Mono-Carbonyl Curcumin Analogues Attenuate Oxidative Stress in Mouse Models. Biomedicines 2022, 10, 2597. [Google Scholar] [CrossRef] [PubMed]
- Domanskyi, A.; Parlato, R. Oxidative Stress in Neurodegenerative Diseases. Antioxidants 2022, 11, 504. [Google Scholar] [CrossRef]
- Popiołek, Ł.; Biernasiuk, A. Synthesis and Investigation of Antimicrobial Activities of Nitrofurazone Analogues Containing Hydrazide-Hydrazone Moiety. Saudi Pharm. J. 2017, 25, 1097–1102. [Google Scholar] [CrossRef]
- Nasr, T.; Bondock, S.; Rashed, H.M.; Fayad, W.; Youns, M.; Sakr, T.M. Novel Hydrazide-Hydrazone and Amide Substituted Coumarin Derivatives: Synthesis, Cytotoxicity Screening, Microarray, Radiolabeling and in Vivo Pharmacokinetic Studies. Eur. J. Med. Chem. 2018, 151, 723–739. [Google Scholar] [CrossRef]
- Küçükgüzel, S.G.; Mazi, A.; Sahin, F.; Oztürk, S.; Stables, J. Synthesis and Biological Activities of Diflunisal Hydrazide-Hydrazones. Eur. J. Med. Chem. 2003, 38, 1005–1013. [Google Scholar] [CrossRef] [PubMed]
- Mir, R.H.; Mir, P.A.; Mohi-Ud-Din, R.; Sabreen, S.; Maqbool, M.; Shah, A.J.; Shenmar, K.; Raza, S.N.; Pottoo, F.H. A Comprehensive Review on Journey of Pyrrole Scaffold Against Multiple Therapeutic Targets. Anticancer Agents Med. Chem. 2022, 22, 3291–3303. [Google Scholar] [CrossRef]
- Tzvetkova, M.; Nocheva, H.; Danalev, D.; Vladimirova, S.; Yaneva, S.; Bocheva, A. Anti-Inflammatory and Analgesic Activity of Newly Synthesized Peptides Including Pyrrole Moiety. Bulg. Chem. Commun. 2017, 49, 218–222. [Google Scholar]
- Rawat, P.; Singh, R.N.; Ranjan, A.; Gautam, A.; Trivedi, S.; Kumar, M. Study of Antimicrobial and Antioxidant Activities of Pyrrole-Chalcones. J. Mol. Struct. 2021, 1228, 129483. [Google Scholar] [CrossRef]
- Tzankova, D.G.; Vladimirova, S.P.; Peikova, L.P.; Georgieva, M.B. Synthesis and Preliminary Antioxidant Activity Evaluation of New Pyrrole Based Aryl Hydrazones. Bulg. Chem. Commun. 2019, 51, 179–185. [Google Scholar]
- Pham, V.H.; Phan, T.P.D.; Phan, D.C.; Vu, B.D. Synthesis and Bioactivity of Hydrazide-Hydrazones with the 1-Adamantyl-Carbonyl Moiety. Molecules 2019, 24, 4000. [Google Scholar] [CrossRef]
- Zdarta, A.; Smułek, W.; Bielan, Z.; Zdarta, J.; Nguyen, L.N.; Zgoła-Grześkowiak, A.; Nghiem, L.D.; Jesionowski, T.; Kaczorek, E. Significance of the Presence of Antibiotics on the Microbial Consortium in Wastewater—The Case of Nitrofurantoin and Furazolidone. Bioresour. Technol. 2021, 339, 125577. [Google Scholar] [CrossRef] [PubMed]
- Devi, J.; Pachwania, S. Synthesis, Characterization, in Vitro Antioxidant and Antimicrobial Activities of Diorganotin(IV) Complexes Derived from Hydrazide Schiff Base Ligands. Phosphorus Sulfur Silicon Relat. Elem. 2021, 196, 1049–1060. [Google Scholar] [CrossRef]
- Coşar, E.; Dincel, E.; Demiray, S.; Sucularlı, E.; Tüccaroğlu, E.; Ozsoy, N.; Ulusoy Güzeldemirci, N. Anticholinesterase Activities of Novel Indole-Based Hydrazide-Hydrazone Derivatives: Design, Synthesis, Biological Evaluation, Molecular Docking Study and in Silico ADME Prediction. J. Mol. Struct. 2021, 1247, 131398. [Google Scholar] [CrossRef]
- Fedorova, T.; Kulikova, O.; Migulin, V.; Muzychuk, O.; Abaimov, D.; Stvolinsky, S. New Derivative of Pyrrole and Carnosine: Synthesis, Physicochemical Properties, and Biological Acivity. Pharm. Chem. J. 2024, 58, 617–624. [Google Scholar] [CrossRef]
- Bhosale, J.D.; Shirolkar, A.R.; Pete, U.D.; Zade, C.M.; Mahajan, D.P.; Hadole, C.D.; Pawar, S.D.; Patil, U.D.; Dabur, R.; Bendre, R. Synthesis, Characterization and Biological Activities of Novel Substituted Formazans of 3, 4-Dimethyl-1H-Pyrrole-2-Carbohydrazide Derivatives. J. Pharm. Res. 2013, 7, 582–587. [Google Scholar] [CrossRef]
- Khalilpour, A.; Asghari, S. Synthesis, Characterization and Evaluation of Cytotoxic and Antioxidant Activities of Dihydropyrimidone Substituted Pyrrole Derivatives. Med. Chem. Res. 2018, 27, 15–22. [Google Scholar] [CrossRef]
- Mateev, E.; Angelov, B.; Kondeva-Burdina, M.; Valkova, I.; Georgieva, M.; Zlatkov, A. Design, Synthesis, Biological Evaluation and Molecular Docking of Pyrrole-Based Compounds as Antioxidant and MAO-B Inhibitory Agents. Farmacia 2022, 70, 344–354. [Google Scholar] [CrossRef]
- Tzankova, D.; Vladimirova, S.; Stefanova, D.; Peikova, L.; Kondeva-Burdina, M.; Georgieva, M. Evaluation of the in Vitro Neurotoxic and Neuroprotective Effects at Cellular and Subcellular Levels of Newly Synthesized N-Pyrrolyl Hydrazones. Farmacia 2022, 70, 872–879. [Google Scholar] [CrossRef]
- Mateev, E.; Kondeva-Burdina, M.; Georgieva, M.; Mateeva, A.; Valkova, I.; Tzankova, V.; Zlatkov, A. Synthesis, Biological Evaluation, Molecular Docking and ADME Studies of Novel Pyrrole-Based Schiff Bases as Dual Acting MAO/AChE Inhibitors. Sci. Pharm. 2024, 92, 18. [Google Scholar] [CrossRef]
- Mateev, E.; Georgieva, M.; Zlatkov, A. Design, Microwave-Assisted Synthesis, Biological Evaluation, Molecular Docking and ADME Studies of Pyrrole-Based Hydrazide-Hydrazones as Potential Antioxidant Agents. Maced. J. Chem. Chem. Eng. 2022, 41, 175–186. [Google Scholar] [CrossRef]
- Di, L.; Artursson, P.; Avdeef, A.; Benet, L.Z.; Houston, J.B.; Kansy, M.; Kerns, E.H.; Lennernäs, H.; Smith, D.A.; Sugano, K. The Critical Role of Passive Permeability in Designing Successful Drugs. ChemMedChem 2020, 15, 1862–1874. [Google Scholar] [CrossRef] [PubMed]
- Nhlapho, S.; Nyathi, M.H.L.; Ngwenya, B.L.; Dube, T.; Telukdarie, A.; Munien, I.; Vermeulen, A.; Chude-Okonkwo, U.A.K. Druggability of Pharmaceutical Compounds Using Lipinski Rules with Machine Learning. Sci. Pharm. 2024, 3, 177–192. [Google Scholar] [CrossRef]
- Pajouhesh, H.; Lenz, G.R. Medicinal Chemical Properties of Successful Central Nervous System Drugs. NeuroRx J. Am. Soc. Exp. Neurother. 2005, 2, 541–553. [Google Scholar] [CrossRef]
- Mateev, E.; Karatchobanov, V.; Dedja, M.; Diamantakos, K.; Mateeva, A.; Muhammed, M.T.; Irfan, A.; Kondeva-Burdina, M.; Valkova, I.; Georgieva, M.; et al. Novel Pyrrole Derivatives as Multi-Target Agents for the Treatment of Alzheimer’s Disease: Microwave-Assisted Synthesis, In Silico Studies and Biological Evaluation. Pharmaceuticals 2024, 17, 1171. [Google Scholar] [CrossRef] [PubMed]
- Van de Waterbeemd, H.; Camenisch, G.; Folkers, G.; Chretien, J.R.; Raevsky, O.A. Estimation of Blood-Brain Barrier Crossing of Drugs Using Molecular Size and Shape, and H-Bonding Descriptors. J. Drug Target. 1998, 6, 151–165. [Google Scholar] [CrossRef]
- Ghose, A.K.; Herbertz, T.; Hudkins, R.L.; Dorsey, B.D.; Mallamo, J.P. Knowledge-Based, Central Nervous System (CNS) Lead Selection and Lead Optimization for CNS Drug Discovery. ACS Chem. Neurosci. 2012, 3, 50–68. [Google Scholar] [CrossRef]
- Reutov, V.P.; Samosudova, N.V.; Sorokina, E.G. A Model of Glutamate Neurotoxicity and Mechanisms of the Development of the Typical Pathological Process. Biophysics 2019, 64, 233–250. [Google Scholar] [CrossRef]
- Coyle, J.T.; Puttfarcken, P. Oxidative Stress, Glutamate, and Neurodegenerative Disorders. Science 1993, 262, 689–695. [Google Scholar] [CrossRef]
- Urso, M.L.; Clarkson, P.M. Oxidative Stress, Exercise, and Antioxidant Supplementation. Toxicology 2003, 189, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Grotto, D.; Maria, L.S.; Valentini, J.; Paniz, C.; Schmitt, G.; Garcia, S.C.; Pomblum, V.J.; Rocha, J.B.T.; Farina, M. Importance of the Lipid Peroxidation Biomarkers and Methodological Aspects FOR Malondialdehyde Quantification. Quím. Nova 2009, 32, 169–174. [Google Scholar] [CrossRef]
- Esterbauer, H.; Cheeseman, K.H. Determination of Aldehydic Lipid Peroxidation Products: Malonaldehyde and 4-Hydroxynonenal. Methods Enzymol. 1990, 186, 407–421. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, D.J.; Capela, J.P.; de Lourdes Bastos, M.; Carvalho, F. In Vitro Models for Neurotoxicology Research. Toxicol. Res. 2015, 4, 801–842. [Google Scholar] [CrossRef]
- Forman, H.J.; Davies, K.J.A.; Ursini, F. How Do Nutritional Antioxidants Really Work: Nucleophilic Tone and Para-Hormesis versus Free Radical Scavenging in Vivo. Free Radic. Biol. Med. 2014, 66, 24–35. [Google Scholar] [CrossRef]
- Esmaeili-Mahani, S.; Vazifekhah, S.; Pasban-Aliabadi, H.; Abbasnejad, M.; Sheibani, V. Protective Effect of Orexin-A on 6-Hydroxydopamine-Induced Neurotoxicity in SH-SY5Y Human Dopaminergic Neuroblastoma Cells. Neurochem. Int. 2013, 63, 719–725. [Google Scholar] [CrossRef]
- Martínez, M.-A.; Rodríguez, J.-L.; Lopez-Torres, B.; Martínez, M.; Martínez-Larrañaga, M.-R.; Maximiliano, J.-E.; Anadón, A.; Ares, I. Use of Human Neuroblastoma SH-SY5Y Cells to Evaluate Glyphosate-Induced Effects on Oxidative Stress, Neuronal Development and Cell Death Signaling Pathways. Environ. Int. 2020, 135, 105414. [Google Scholar] [CrossRef]
- Drahota, Z.; Kriváková, P.; Cervinková, Z.; Kmonícková, E.; Lotková, H.; Kucera, O.; Houstek, J. Tert-Butyl Hydroperoxide Selectively Inhibits Mitochondrial Respiratory-Chain Enzymes in Isolated Rat Hepatocytes. Physiol. Res. 2005, 54, 67–72. [Google Scholar] [CrossRef]
- O’Donnell, V.; Burkitt, M.J. Mitochondrial Metabolism of a Hydroperoxide to Free Radicals in Human Endothelial Cells: An Electron Spin Resonance Spin-Trapping Investigation. Biochem. J. 1994, 304 Pt 3, 707–713. [Google Scholar] [CrossRef]
- Ollinger, K.; Brunk, U.T. Cellular Injury Induced by Oxidative Stress Is Mediated through Lysosomal Damage. Free Radic. Biol. Med. 1995, 19, 565–574. [Google Scholar] [CrossRef]
- Kehrer, J.P. The Haber-Weiss Reaction and Mechanisms of Toxicity. Toxicology 2000, 149, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, M.A.; Diab, K.A.; Abdel-Samie, N.S.; Omara, E.A.; Hassan, Z.M. Carbon Tetrachloride Induced Hepato/Renal Toxicity in Experimental Mice: Antioxidant Potential of Egyptian Salvia Officinalis L Essential Oil. Environ. Sci. Pollut. Res. 2018, 25, 27858–27876. [Google Scholar] [CrossRef]
- Daina, A.; Zoete, V. A BOILED-Egg To Predict Gastrointestinal Absorption and Brain Penetration of Small Molecules. ChemMedChem 2016, 11, 1117–1121. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A Free Web Tool to Evaluate Pharmacokinetics, Drug-Likeness and Medicinal Chemistry Friendliness of Small Molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed]
- Taupin, P.; Ben-Ari, Y.; Roisin, M.-P. Subcellular Fractionation on Percoll Gradient of Mossy Fiber Synaptosomes: Evoked Release of Glutamate, GABA, Aspartate and Glutamate Decar☐ ylase Activity in Control and Degranulated Rat Hippocampus. Brain Res. 1994, 644, 313–321. [Google Scholar] [CrossRef]
- Sims, N.R.; Anderson, M.F. Isolation of Mitochondria from Rat Brain Using Percoll Density Gradient Centrifugation. Nat. Protoc. 2008, 3, 1228–1239. [Google Scholar] [CrossRef] [PubMed]
- Stokes, A.H.; Freeman, W.M.; Mitchell, S.G.; Burnette, T.A.; Hellmann, G.M.; Vrana, K.E. Induction of GADD45 and GADD153 in Neuroblastoma Cells by Dopamine-Induced Toxicity. NeuroToxicology 2002, 23, 675–684. [Google Scholar] [CrossRef]
- Kondeva-Burdina, M.; Voynova, M.; Shkondrov, A.; Aluani, D.; Tzankova, V.; Krasteva, I. Effects of Amanita muscaria Extract on Different in Vitro Neurotoxicity Models at Subcellular and Cellular Levels. Food Chem. Toxicol. 2019, 132, 110687. [Google Scholar] [CrossRef]
- Peña, F.; Gutiérrez-Lerma, A.; Quiroz-Baez, R.; Arias, C. The Role of β-Amyloid Protein in Synaptic Function: Implications for Alzheimer’s Disease Therapy. Curr. Neuropharmacol. 2006, 4, 149–163. [Google Scholar] [CrossRef]
- Robyt, J.F.; Ackerman, R.J.; Chittenden, C.G. Reaction of Protein Disulfide Groups with Ellman’s Reagent: A Case Study of the Number of Sulfhydryl and Disulfide Groups in Aspergillus oryzae α-Amylase, Papain, and Lysozyme. Arch. Biochem. Biophys. 1971, 147, 262–269. [Google Scholar] [CrossRef]
- Karlsson, J.; Emgard, M.; Brundin, P.; Burkitt, M.J. Trans-Resveratrol Protects Embryonic Mesencephalic Cells from Tert-Butyl Hydroperoxide: Electron Paramagnetic Resonance Spin Trapping Evidence for a Radical Scavenging Mechanism. J. Neurochem. 2000, 75, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Shirani, M.; Alizadeh, S.; Mahdavinia, M.; Dehghani, M.A. The Ameliorative Effect of Quercetin on Bisphenol A-Induced Toxicity in Mitochondria Isolated from Rats. Environ. Sci. Pollut. Res. Int. 2019, 26, 7688–7696. [Google Scholar] [CrossRef] [PubMed]
- Ravindranath, V.; Anandatheerthavarada, H.K. Preparation of Brain Microsomes with Cytochrome P450 Activity Using Calcium Aggregation Method. Anal. Biochem. 1990, 187, 310–313. [Google Scholar] [CrossRef]
- Mansuy, D.; Sassi, A.; Dansette, P.M.; Plat, M. A New Potent Inhibitor of Lipid Peroxidation in Vitro and in Vivo, the Hepatoprotective Drug Anisyldithiolthione. Biochem. Biophys. Res. Commun. 1986, 135, 1015–1021. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]


| ID | MW | pKa | logP | logD7.4 | TPSA | RB | HBD | HBA | ROF |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 509.40 | pKa1 2.4 pKa2 11.17 | 3.02 | 3.02 | 102.14 | 9 | 3 | 4 | 1 |
| 7 | 687.58 | 10.79 | 3.10 | 3.10 | 116.17 | 14 | 2 | 7 | 1 |
| 9 | 654.55 | 11.14 | 3.50 | 3.50 | 117.58 | 13 | 3 | 5 | 1 |
| 11 | 632.46 | 10.66 | 2.89 | 2.89 | 147.44 | 12 | 2 | 7 | 1 |
| 12 | 613.5 | 8.38 | 3.57 | 3.53 | 108.71 | 11 | 3 | 5 | 1 |
| 14 | 647.56 | 11.13 | 4.65 | 4.65 | 88.48 | 11 | 2 | 4 | 1 |
| 15 | 697.62 | 11.13 | 5.17 | 5.17 | 88.48 | 11 | 2 | 4 | 2 |
| Compounds | IC50 (µM) | 95% Confidence Interval |
|---|---|---|
| 1 | 53 | 44.5–61.2 |
| 7 | >500 | NA |
| 9 | >500 | NA |
| 11 | 263.5 | 251.3–274.5 |
| 12 | >500 | NA |
| 14 | >500 | NA |
| 15 | >500 | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stefanova, D.; Garip, A.; Mateev, E.; Kondeva-Burdina, M.; Yordanov, Y.; Tzankova, D.; Mateeva, A.; Valkova, I.; Georgieva, M.; Zlatkov, A.; et al. Antioxidant and Neuroprotective Properties of Selected Pyrrole-Containing Azomethine Compounds in Neurotoxicity Models In Vitro. Int. J. Mol. Sci. 2025, 26, 3957. https://doi.org/10.3390/ijms26093957
Stefanova D, Garip A, Mateev E, Kondeva-Burdina M, Yordanov Y, Tzankova D, Mateeva A, Valkova I, Georgieva M, Zlatkov A, et al. Antioxidant and Neuroprotective Properties of Selected Pyrrole-Containing Azomethine Compounds in Neurotoxicity Models In Vitro. International Journal of Molecular Sciences. 2025; 26(9):3957. https://doi.org/10.3390/ijms26093957
Chicago/Turabian StyleStefanova, Denitsa, Alime Garip, Emilio Mateev, Magdalena Kondeva-Burdina, Yordan Yordanov, Diana Tzankova, Alexandrina Mateeva, Iva Valkova, Maya Georgieva, Alexander Zlatkov, and et al. 2025. "Antioxidant and Neuroprotective Properties of Selected Pyrrole-Containing Azomethine Compounds in Neurotoxicity Models In Vitro" International Journal of Molecular Sciences 26, no. 9: 3957. https://doi.org/10.3390/ijms26093957
APA StyleStefanova, D., Garip, A., Mateev, E., Kondeva-Burdina, M., Yordanov, Y., Tzankova, D., Mateeva, A., Valkova, I., Georgieva, M., Zlatkov, A., & Tzankova, V. (2025). Antioxidant and Neuroprotective Properties of Selected Pyrrole-Containing Azomethine Compounds in Neurotoxicity Models In Vitro. International Journal of Molecular Sciences, 26(9), 3957. https://doi.org/10.3390/ijms26093957








