The Influence of an Acute Endurance Intervention on Breast Cancer Cell Growth—A Pilot Study
Abstract
:1. Introduction
2. Results
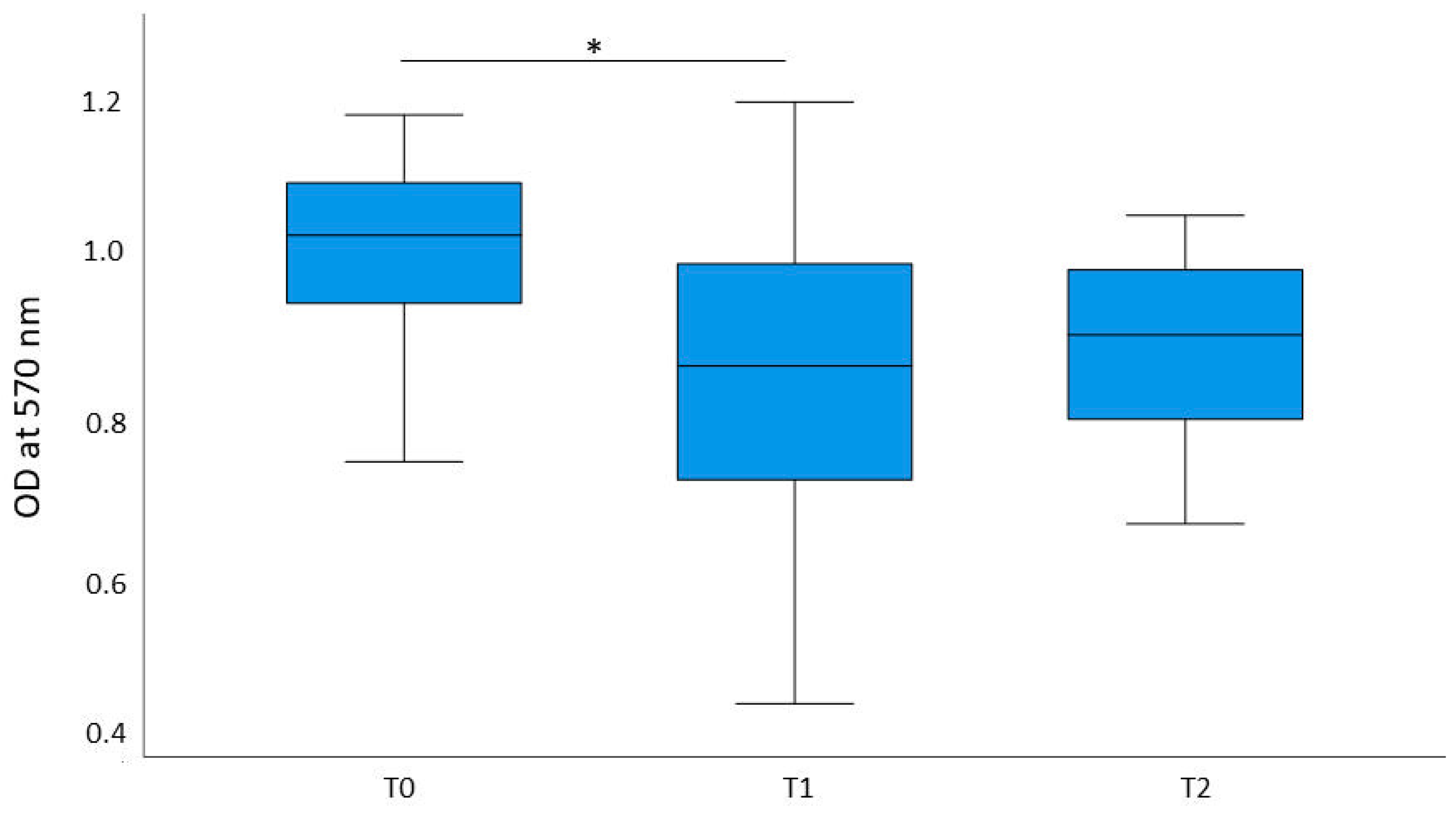
2.1. Cell Proliferation
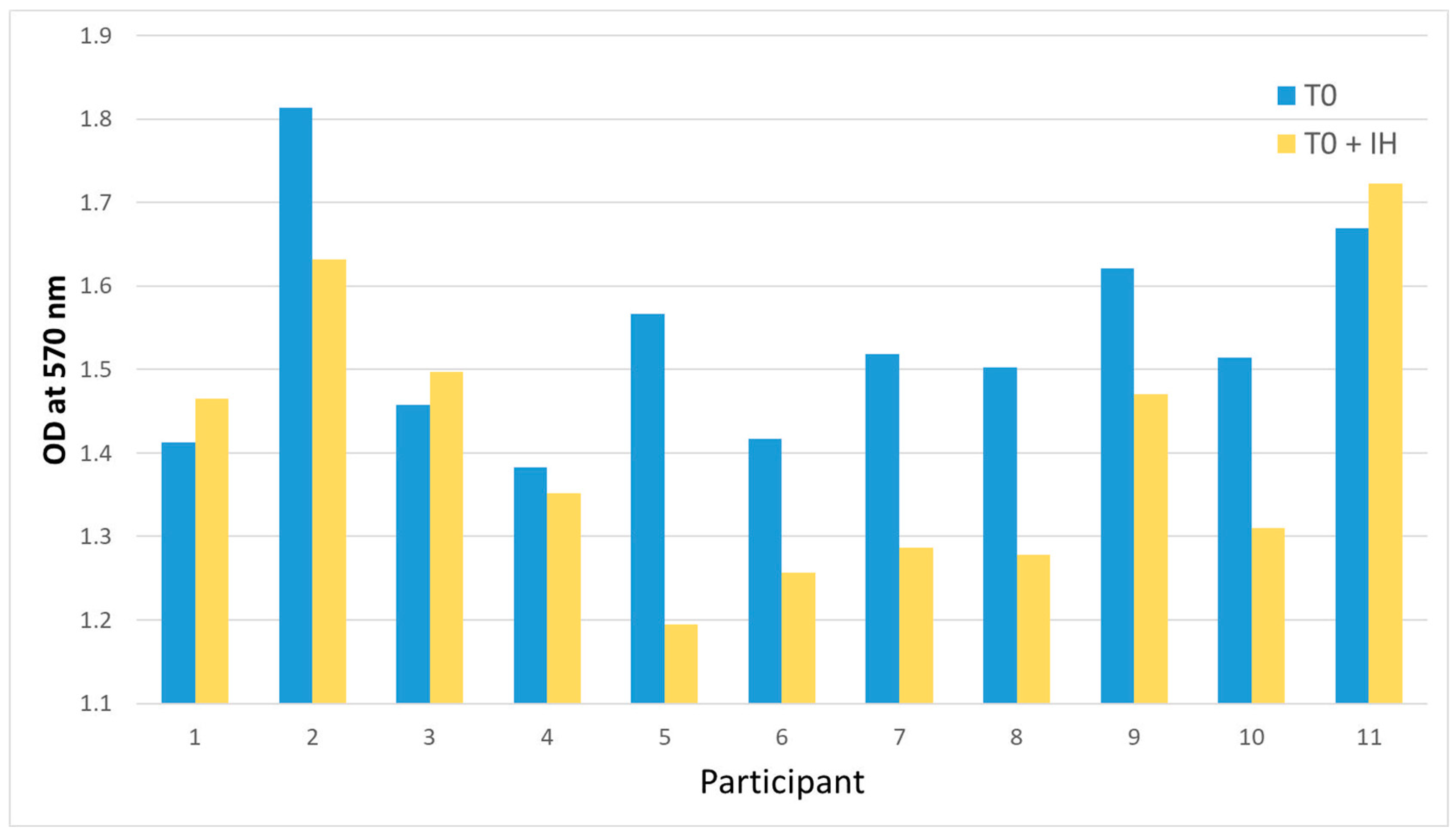
2.2. Analysis of the Cytotoxic Effects
2.3. Cytokine Array
2.4. Blocking CXCR3 with AMG 487
2.4.1. Analysis of the Cytotoxic Effects
2.4.2. Proliferation
3. Discussion
Limitations
4. Materials and Methods
4.1. Participants and Study Design
4.2. Intervention
4.3. Analysis of the Cytotoxic Effects
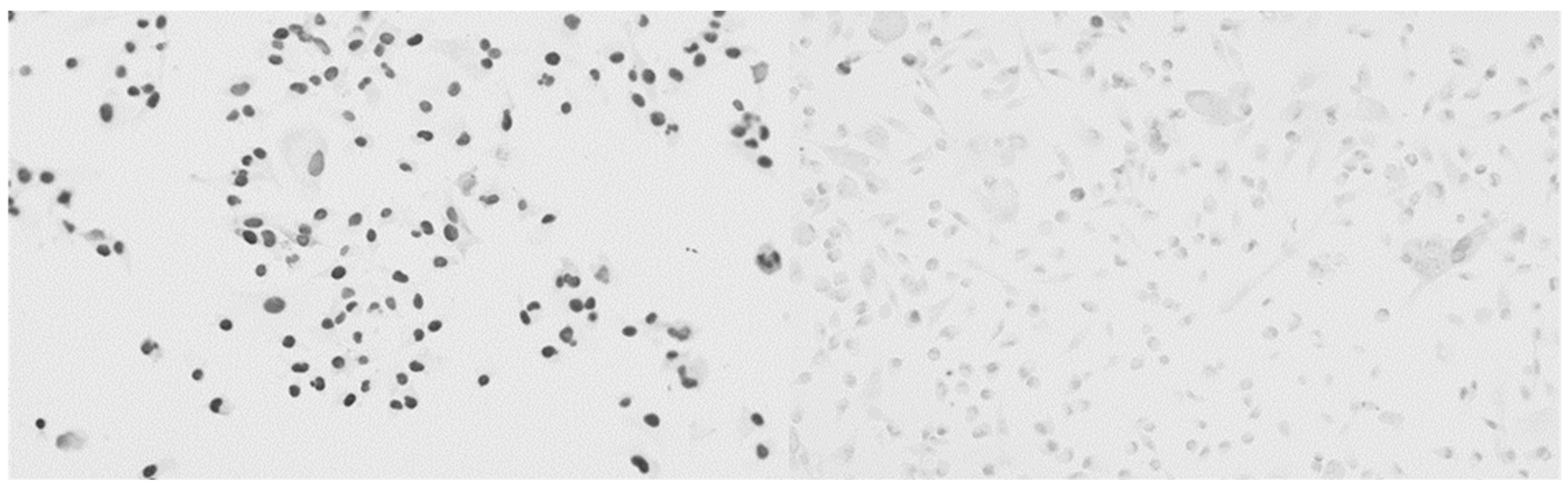
4.4. Immunohistochemistry
4.5. Cytokine Array
4.6. Lactate Concentration
4.7. Blocking CXCL9
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Natural killer cells | NK cells |
| Tumor microenvironment | TME |
| Myeloid-derived suppressor cells | MDSCs |
| Interleukin-6 | IL-6 |
| Vascular endothelial growth factor | VEGF |
| Matrix metalloprotease | MMP |
| Physical Activity Readiness Questionnaire | PAR-Q |
| International Physical Activity Questionnaire | IPAQ |
| Cardio Pulmonary Exercise Testing | CEPT |
| Peak aerobic capacity | VO2peak |
| Rate of perceived exertion | RPE |
| Phosphate-buffered saline | PBS |
| TRIS-buffered saline | TBS |
| Bovine albumin | BSA |
| 3,3′Diaminobenzidine | DAB |
| Chemokine C-X-C motif ligand 9 | CXCL9 |
| CC-chemokine ligand | 15 CCL15 |
| Intraclass correlation coefficient | ICC |
| Inhibitor | IH |
| C-X-C motif chemokine 10 | CXCL10 |
| C-X-C motif chemokine 11 | CXCL11 |
Appendix A
| Variable | Mean | SD 5 |
|---|---|---|
| Age in years | 46.4 | 9.4 |
| Weight in kg | 66.2 | 10.2 |
| Height in cm | 167.9 | 7.3 |
| IPAQ-SF category 1 | Minimally active N = 8 Inactive N = 3 | |
| PAR-Q score 2 | 0.1 | 0.3 |
| VO2peak in mL/min/kg 3 | 31.1 | 5.66 |
| 65% VO2peak in mL/min/kg | 20.2 | 3.68 |
| 50% VO2peak in mL/min/kg | 15.6 | 2.83 |
| RPEmax 4 | 19.7 | 0.45 |
| Wattmax | 155.9 | 27.49 |
| Watt65% | 101.3 | 17.87 |
| Watt50% | 78.0 | 13.74 |
| Protein | difT0-T1 | difT1-T2 | difT0-T2 | difRT0-T1 | difRT1-T2 | difRT0-T2 |
|---|---|---|---|---|---|---|
| Leptin | 1466.07 | 0.31 | 453.36 | 0.943 | 667,987 | 629,833 |
| PDGF-BB | 1875.27 | 0.66 | 1233.71 | 702,465 | 0.716 | 502,653 |
| CCL5 | 1.21 | 0.66 | 0.80 | 713,666 | 1.044 | 744,822 |
| MCP1 | 1.42 | 0.64 | 0.91 | 793,319 | 0.773 | 613,088 |
| IL-15 | 0.63 | 0.87 | 0.55 | 1,061,224 | 0.893 | 947,585 |
| Angiogenin | 0.80 | 0.95 | 0.77 | 689,367 | 1.113 | 767,531 |
| BDNF | 0.78 | 0.74 | 0.57 | 550,514 | 1.014 | 558,105 |
| TNF alpha | 0.72 | 0.68 | 0.49 | 1,608 | 1.084 | 1.743 |
| MIG (CX CL9) | 0.92 | 0.72 | 0.66 | 0.497 | 1.332 | 0.662 |
| IL-3 | 0.59 | 0.74 | 0.43 | 0.658 | 1.145 | 0.754 |
| MIP-1delta (CCL 15) | 0.92 | 0.65 | 0.60 | 0.700 | 1.565 | 1.096 |
| IGFBP-1 | 1.26 | 1.05 | 1.33 | 0.721 | 1.070 | 0.771 |
| NAP-2 | 1.27 | 0.49 | 0.62 | 0.996 | 1.096 | 1.092 |
| EGF | 1.25 | 1.31 | 1.63 | 0.602 | 0.704 | 0.424 |
| IGFBP-2 | 1.58 | 0.80 | 1.26 | 0.689 | 1.027 | 0.708 |
| Eotaxin-1 (CCL11) | 0.72 | 1.66 | 1.20 | 0.741 | 1.017 | 0.754 |
| ICAM-1(CD54) | 0.99 | 1.50 | 1.48 | 2.523 | 0.853 | 2.152 |
| TIMP-1 | 1.62 | 0.89 | 1.43 | 0.454 | 1.189 | 0.540 |
| EGFR | 1.43 | 0.71 | 1.02 | 0.761 | 0.948 | 0.721 |
| TIMP-2 | 1.46 | 0.84 | 1.23 | 0.782 | 1.091 | 0.853 |
| ENA-78 (CXCL5) | 1.75 | 0.56 | 0.98 | 0.763 | 1.029 | 0.785 |
| IGFBP-6 | 1.45 | 0.92 | 1.33 | 0.880 | 0.875 | 0.770 |
| MIP-1-beta | 1.61 | 0.81 | 1.31 | 0.654 | 0.658 | 0.430 |
| TRAILR3 | 0.07 | 0.48 | 0.04 | 1.355 | 1.032 | 1.398 |
| Adiponectin | 19.78 | 1.04 | 20.50 | 0.854 | 0.997 | 0.852 |
| MIP-3-beta | 2.05 | 0.81 | 1.67 | 0.562 | 0.939 | 0.528 |
| MSP | 1.44 | 0.85 | 1.22 | 0.497 | 0.951 | 0.473 |
| uPAR | 1.35 | 0.70 | 0.95 | 1.016 | 0.615 | 0.625 |
| ANGPT2 | 1.22 | 1.49 | 1.82 | 0.649 | 1.396 | 0.907 |
| OPG | 0.59 | 0.99 | 0.58 | 0.580 | 1.096 | 0.636 |
| GRO | 0.54 | 1.17 | 0.64 | 0.605 | 0.985 | 0.596 |
| gp130 | 2.35 | 0.96 | 2.26 | 0.387 | 0.965 | 0.374 |
| HCC-4 | 0.32 | 1.39 | 0.44 | 0.585 | 0.847 | 0.496 |
| Il-6 R | 2788.88 | 1.19 | 3321.07 | 0.878 | 0.916 | 0.804 |
References
- Westphal, T.; Gampenrieder, S.P.; Rinnerthaler, G.; Greil, R. Cure in metastatic breast cancer. Memo-Mag. Eur. Med. Oncol. 2018, 11, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.C.; Lee, I.-M.; Weiderpass, E.; Campbell, P.T.; Sampson, J.N.; Kitahara, C.M.; Keadle, S.K.; Arem, H.; Berrington de Gonzalez, A.; Hartge, P.; et al. Association of Leisure-Time Physical Activity With Risk of 26 Types of Cancer in 1.44 Million Adults. JAMA Intern. Med. 2016, 176, 816–825. [Google Scholar] [CrossRef]
- Mock, V.; Frangakis, C.; Davidson, N.E.; Ropka, M.E.; Pickett, M.; Poniatowski, B.; Stewart, K.J.; Cameron, L.; Zawacki, K.; Podewils, L.J.; et al. Exercise manages fatigue during breast cancer treatment: A randomized controlled trial. J. Psychol. Soc. Behav. Dimens. Cancer 2005, 14, 464–477. [Google Scholar] [CrossRef] [PubMed]
- Dieli-Conwright, C.M.; Orozco, B.Z. Exercise after breast cancer treatment: Current perspectives. Breast Cancer Targets Ther. 2015, 7, 353–362. [Google Scholar] [CrossRef] [PubMed]
- De Lima, C.; Alves, L.E.; Iagher, F.; Machado, A.F.; Bonatto, S.J.; Kuczera, D.; de Souza, C.F.; Pequito, D.C.; Muritiba, A.L.; Nunes, E.A.; et al. Anaerobic exercise reduces tumor growth, cancer cachexia and increases macrophage and lymphocyte response in Walker 256 tumor-bearing rats. Eur. J. Appl. Physiol. 2008, 104, 957–964. [Google Scholar] [CrossRef]
- Padilha, C.S.; Testa, M.T.; Marinello, P.C.; Cella, P.S.; Voltarelli, F.A.; Frajacomo, F.T.; Cechini, R.; Duarte, J.A.R.; Guarnier, F.A.; Deminice, R. Resistance Exercise Counteracts Tumor Growth in Two Carcinoma Rodent Models. Med. Sci. Sports Exerc. 2019, 51, 2003–2011. [Google Scholar] [CrossRef]
- Verma, V.K.; Singh, V.; Singh, M.P.; Singh, S.M. Effect of physical exercise on tumor growth regulating factors of tumor microenvironment: Implications in exercise-dependent tumor growth retardation. Immunopharmacol. Immunotoxicol. 2009, 31, 274–282. [Google Scholar] [CrossRef]
- Rundqvist, H.; Veliça, P.; Barbieri, L.; Gameiro, P.A.; Bargiela, D.; Gojkovic, M.; Mijwel, S.; Reitzner, S.M.; Wulliman, D.; Ahlstedt, E.; et al. Cytotoxic T-cells mediate exercise-induced reductions in tumor growth. eLife 2020, 9, e59996. [Google Scholar] [CrossRef]
- Carter, S.; Solomon, T.P.J. In vitro experimental models for examining the skeletal muscle cell biology of exercise: The possibilities, challenges and future developments. Pflug. Arch. Eur. J. Physiol. 2019, 471, 413–429. [Google Scholar] [CrossRef]
- Fiuza-Luces, C.; Valenzuela, P.L.; Gálvez, B.G.; Ramírez, M.; López-Soto, A.; Simpson, R.J.; Lucia, A. The effect of physical exercise on anticancer immunity. Nat. Rev. Immunol. 2024, 24, 282–293. [Google Scholar] [CrossRef]
- Lavín-Pérez, A.M.; Collado-Mateo, D.; Abbasi, S.; Ferreira-Júnior, J.B.; Hekmatikar, A.H.A. Effects of exercise on immune cells with tumor-specific activity in breast cancer patients and survivors: A systematic review and meta-analysis. Support. Care Cancer 2023, 31, 507. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, L.; Christensen, J.F.; Hojman, P. Effects of exercise on tumor physiology and metabolism. Cancer J. (Sudbury Mass.) 2015, 21, 111–116. [Google Scholar] [CrossRef]
- Spiliopoulou, P.; Gavriatopoulou, M.; Kastritis, E.; Dimopoulos, M.A.; Terzis, G. Exercise-Induced Changes in Tumor Growth via Tumor Immunity. Sports 2021, 9, 46. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, L.; Hojman, P. Muscle-to-organ cross talk mediated by myokines. Adipocyte 2012, 1, 164–167. [Google Scholar] [CrossRef]
- Wiggins, J.M.; Opoku-Acheampong, A.B.; Baumfalk, D.R.; Siemann, D.W.; Behnke, B.J. Exercise and the Tumor Microenvironment: Potential Therapeutic Implications. Exerc. Sport. Sci. Rev. 2018, 46, 56–64. [Google Scholar] [CrossRef]
- Gunasekara, N.; Clauss, D.; Bloch, W. Effects of exercise-induced changes in myokine expression on the tumor microenvironment. Sports Med. Int. Open 2024, 8, a22831663. [Google Scholar] [CrossRef]
- Balkwill, F.R.; Capasso, M.; Hagemann, T. The tumor microenvironment at a glance. J. Cell Sci. 2012, 125, 5591–5596. [Google Scholar] [CrossRef]
- Pedersen, B.K. Exercise-induced myokines and their role in chronic diseases. Brain Behav. Immun. 2011, 25, 811–816. [Google Scholar] [CrossRef]
- Natarajan, A.; Pradhan, R.; Dieterich, W.; Schwappacher, R.; Reljic, D.; Herrmann, H.J.; Neurath, M.F.; Hack, C.C.; Beckmann, M.W.; Zopf, Y. The Influence of Physical Training on Breast Cancer: The Role of Exercise-Induced Myokines in Regulating Breast Cancer Cell Growth and Survival. Int. J. Mol. Sci. 2024, 25, 11379. [Google Scholar] [CrossRef]
- Hoffmann, C.; Weigert, C. Skeletal Muscle as an Endocrine Organ: The Role of Myokines in Exercise Adaptations. Cold Spring Harb. Perspect. Med. 2017, 7, a029793. [Google Scholar] [CrossRef]
- Hofmann, P. Cancer and Exercise: Warburg Hypothesis, Tumour Metabolism and High-Intensity Anaerobic Exercise. Sports 2018, 6, 10. [Google Scholar] [CrossRef] [PubMed]
- Shalamzari, S.A.; Agha-Alinejad, H.; Alizadeh, S.; Shahbazi, S.; Khatib, Z.K.; Kazemi, A.; Saei, M.A.; Minayi, N. The effect of exercise training on the level of tissue IL-6 and vascular endothelial growth factor in breast cancer bearing mice. Iran. J. Basic. Med. Sci. 2014, 17, 231–258. [Google Scholar]
- Murphy, E.A.; Davis, J.M.; Barrilleaux, T.L.; McClellan, J.L.; Steiner, J.L.; Carmichael, M.D.; Pena, M.M.; Hebert, J.R.; Green, J.E. Benefits of exercise training on breast cancer progression and inflammation in C3(1)SV40Tag mice. Cytokine 2011, 55, 274–279. [Google Scholar] [CrossRef]
- Farley, M.J.; Boytar, A.N.; Adlard, K.N.; Salisbury, C.E.; Hart, N.H.; Schaumberg, M.A.; Jenkins, D.G.; Skinner, T.L. Interleukin-15 and high-intensity exercise: Relationship with inflammation, body composition and fitness in cancer survivors. J. Physiol. 2024, 602, 5203–5215. [Google Scholar] [CrossRef] [PubMed]
- Bloch, W. Tumour muscle crosstalk more as regulation of muscle wasting—Role of exercise. Acta Physiol. 2017, 219, 704–705. [Google Scholar] [CrossRef]
- Bao, J.-M.; Dang, Q.; Lin, C.-J.; Lo, U.-G.; Feldkoren, B.; Dang, A.; Hernandez, E.; Li, F.; Panwar, V.; Lee, C.-F.; et al. SPARC is a key mediator of TGF-β-induced renal cancer metastasis. J. Cell Physiol. 2021, 236, 1926–1938. [Google Scholar] [CrossRef] [PubMed]
- Rundqvist, H.; Augsten, M.; Strömberg, A.; Rullman, E.; Mijwel, S.; Kharaziha, P.; Panaretakis, T.; Gustafsson, T.; Östman, A. Effect of acute exercise on prostate cancer cell growth. PLoS ONE 2013, 8, e67579. [Google Scholar] [CrossRef]
- Ishihara, S.; Hata, K.; Hirose, K.; Okui, T.; Toyosawa, S.; Uzawa, N.; Nishimura, R.; Yoneda, T. The lactate sensor GPR81 regulates glycolysis and tumor growth of breast cancer. Sci. Rep. 2022, 12, 6261. [Google Scholar] [CrossRef]
- Apostolova, P.; Pearce, E.L. Lactic acid and lactate: Revisiting the physiological roles in the tumor microenvironment. Trends Immunol. 2022, 43, 969–977. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Q.; Huang, X.; Yang, M.; Zhou, S.; Li, Z.; Fang, Z.; Tang, Y.; Chen, Q.; Hou, H.; et al. Lactate in the tumor microenvironment: A rising star for targeted tumor therapy. Front. Nutr. 2023, 10, 1113739. [Google Scholar] [CrossRef]
- Huang, Q.; Wu, M.; Wu, X.; Zhang, Y.; Xia, Y. Muscle-to-tumor crosstalk: The effect of exercise-induced myokine on cancer progression. Biochim. Biophys. Acta Rev. Cancer 2022, 1877, 188761. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Lawrence Erlbaum Associates: New York, NY, USA, 1988. [Google Scholar]
- Pan, M.; Wei, X.; Xiang, X.; Liu, Y.; Zhou, Q.; Yang, W. Targeting CXCL9/10/11-CXCR3 axis: An important component of tumor-promoting and antitumor immunity. Clin. Transl. Oncol. 2023, 25, 2306–2320. [Google Scholar] [CrossRef] [PubMed]
- Ngo-Huang, A.; Fricke, B.C.; Schadler, K.L.; Parker, N.H. Preliminary Evidence on the Effects of Exercise on Tumor Biology: A Potential Guide for Prescribing Exercise. Curr. Phys. Med. Rehabil. Rep. 2021, 9, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Shi, X.; Yu, Z.; Ma, X. High-intensity interval training in breast cancer patients: A systematic review and meta-analysis. Cancer Med. 2023, 12, 17692–17705. [Google Scholar] [CrossRef]
- Ashcraft, K.A.; Peace, R.M.; Betof, A.S.; Dewhirst, M.W.; Jones, L.W. Efficacy and Mechanisms of Aerobic Exercise on Cancer Initiation, Progression, and Metastasis: A Critical Systematic Review of In Vivo Preclinical Data. Cancer Res. 2016, 76, 4032–4050. [Google Scholar] [CrossRef]
- Ferioli, M.; Zauli, G.; Maiorano, P.; Milani, D.; Mirandola, P.; Neri, L.M. Role of physical exercise in the regulation of epigenetic mechanisms in inflammation, cancer, neurodegenerative diseases, and aging process. J. Cell Physiol. 2019, 234, 14852–14864. [Google Scholar] [CrossRef]
- Eschke, R.-C.K.-R.; Lampit, A.; Schenk, A.; Javelle, F.; Steindorf, K.; Diel, P.; Bloch, W.; Zimmer, P. Impact of Physical Exercise on Growth and Progression of Cancer in Rodents-A Systematic Review and Meta-Analysis. Front. Oncol. 2019, 9, 35. [Google Scholar] [CrossRef]
- Esteves, M.; Monteiro, M.P.; Duarte, J.A. Role of Regular Physical Exercise in Tumor Vasculature: Favorable Modulator of Tumor Milieu. Int. J. Sports Med. 2021, 42, 389–406. [Google Scholar] [CrossRef]
- Dethlefsen, C.; Pedersen, K.S.; Hojman, P. Every exercise bout matters: Linking systemic exercise responses to breast cancer control. Breast Cancer Res. Treat. 2017, 162, 399–408. [Google Scholar] [CrossRef]
- Lei, X.; Lei, Y.; Li, J.-K.; Du, W.-X.; Li, R.-G.; Yang, J.; Li, J.; Li, F.; Tan, H.-B. Immune cells within the tumor microenvironment: Biological functions and roles in cancer immunotherapy. Cancer Lett. 2020, 470, 126–133. [Google Scholar] [CrossRef]
- Dethlefsen, C.; Lillelund, C.; Midtgaard, J.; Andersen, C.; Pedersen, B.K.; Christensen, J.F.; Hojman, P. Exercise regulates breast cancer cell viability: Systemic training adaptations versus acute exercise responses. Breast Cancer Res. Treat. 2016, 159, 469–479. [Google Scholar] [CrossRef]
- Brown, J.C.; Spielmann, G.; Yang, S.; Compton, S.L.E.; Jones, L.W.; Irwin, M.L.; Ligibel, J.A.; Meyerhardt, J.A. Effects of exercise or metformin on myokine concentrations in patients with breast and colorectal cancer: A phase II multi-centre factorial randomized trial. J. Cachexia Sarcopenia Muscle 2024, 15, 1520–1527. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Tomás, R.; Pérez-Guillén, I. Lactate in the Tumor Microenvironment: An Essential Molecule in Cancer Progression and Treatment. Cancers 2020, 12, 3244. [Google Scholar] [CrossRef]
- Mortazavi Farsani, S.S.; Verma, V. Lactate mediated metabolic crosstalk between cancer and immune cells and its therapeutic implications. Front. Oncol. 2023, 13, 1175532. [Google Scholar] [CrossRef]
- Liu, C.; Luo, D.; Reynolds, B.A.; Meher, G.; Katritzky, A.R.; Lu, B.; Gerard, C.J.; Bhadha, C.P.; Harrison, J.K. Chemokine receptor CXCR3 promotes growth of glioma. Carcinogenesis 2011, 32, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Lacotte, S.; Brun, S.; Muller, S.; Dumortier, H. CXCR3, inflammation, and autoimmune diseases. Ann. N. Y. Acad. Sci. 2009, 1173, 310–317. [Google Scholar] [CrossRef]
- Sun, W.-H.; Peng, T.-J.; Tang, S.-J.; Lin, J.-Y.; Wang, C.-Y.; Fang, H.-J.; Sun, K.-H. CXCR3 isoform A promotes head and neck cancer progression by enhancing stem-like property and chemoresistance. J. Oral. Pathol. Med. 2022, 51, 791–800. [Google Scholar] [CrossRef]
- Tokunaga, R.; Zhang, W.; Naseem, M.; Puccini, A.; Berger, M.D.; Soni, S.; McSkane, M.; Baba, H.; Lenz, H.-J. CXCL9, CXCL10, CXCL11/CXCR3 axis for immune activation—A target for novel cancer therapy. Cancer Treat. Rev. 2018, 63, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Reader, J.C.; Ma, X.; Kundu, N.; Kochel, T.; Fulton, A.M. Divergent roles of CXCR3 isoforms in promoting cancer stem-like cell survival and metastasis. Breast Cancer Res. Treat. 2015, 149, 403–415. [Google Scholar] [CrossRef]
- Saleem, A.; Hood, D.A. Acute exercise induces tumour suppressor protein p53 translocation to the mitochondria and promotes a p53-Tfam-mitochondrial DNA complex in skeletal muscle. J. Physiol. 2013, 591, 3625–3636. [Google Scholar] [CrossRef]
- Saleem, A.; Carter, H.N.; Hood, D.A. p53 is necessary for the adaptive changes in cellular milieu subsequent to an acute bout of endurance exercise. Am. J. Physiol. Am. J. Physiol. Cell Physiol. 2014, 306, C241–C249. [Google Scholar] [CrossRef] [PubMed]
- Guo, K.; Feng, G.; Yan, Q.; Sun, L.; Zhang, K.; Shen, F.; Shen, M.; Ruan, S. CXCR4 and CXCR3 are two distinct prognostic biomarkers in breast cancer: Database mining for CXCR family members. Mol. Med. Rep. 2019, 20, 4791–4802. [Google Scholar] [CrossRef]
- Wu, Z.; Huang, X.; Han, X.; Li, Z.; Zhu, Q.; Yan, J.; Yu, S.; Jin, Z.; Wang, Z.; Zheng, Q.; et al. The chemokine CXCL9 expression is associated with better prognosis for colorectal carcinoma patients. Biomed. Pharmacother. 2016, 78, 8–13. [Google Scholar] [CrossRef]
- Gomes-Santos, I.L.; Amoozgar, Z.; Kumar, A.S.; Ho, W.W.; Roh, K.; Talele, N.P.; Curtis, H.; Kawaguchi, K.; Jain, R.K.; Fukumura, D. Exercise Training Improves Tumor Control by Increasing CD8+ T-cell Infiltration via CXCR3 Signaling and Sensitizes Breast Cancer to Immune Checkpoint Blockade. Cancer Immunol. Res. 2021, 9, 765–778. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.; Lu, P.; Xia, Y.; Ding, S.; Fan, Y.; Li, X.; Han, P.; Liu, J.; Tian, D.; Liu, M. CXCL9: Evidence and contradictions for its role in tumor progression. Cancer Med. 2016, 5, 3246–3259. [Google Scholar] [CrossRef]
- Bianchi, A.; Marchetti, L.; Hall, Z.; Lemos, H.; Vacca, M.; Paish, H.; Green, K.; Elliott, B.; Tiniakos, D.; Passos, J.F.; et al. Moderate Exercise Inhibits Age-Related Inflammation, Liver Steatosis, Senescence, and Tumorigenesis. J. Immunol. 2021, 206, 904–916. [Google Scholar] [CrossRef] [PubMed]
- Mudry, J.M.; Alm, P.S.; Erhardt, S.; Goiny, M.; Fritz, T.; Caidahl, K.; Zierath, J.R.; Krook, A.; Wallberg-Henriksson, H. Direct effects of exercise on kynurenine metabolism in people with normal glucose tolerance or type 2 diabetes. Diabetes Metab. Res. Rev. 2016, 32, 754–761. [Google Scholar] [CrossRef]
- Schenk, A.; Esser, T.; Belen, S.; Gunasekara, N.; Joisten, N.; Winker, M.T.; Weike, L.; Bloch, W.; Heidenreich, A.; Herden, J.; et al. Distinct distribution patterns of exercise-induced natural killer cell mobilization into the circulation and tumor tissue of patients with prostate cancer. Am. J. Physiol. Cell Physiol. 2022, 323, C879–C884. [Google Scholar] [CrossRef]
- Walser, T.C.; Rifat, S.; Ma, X.; Kundu, N.; Ward, C.; Goloubeva, O.; Johnson, M.G.; Medina, J.C.; Collins, T.L.; Fulton, A.M. Antagonism of CXCR3 Inhibits Lung Metastasis in a Murine Model of Metastatic Breast Cancer. Cancer Res. 2006, 66, 7701–7707. [Google Scholar] [CrossRef]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef]


| Time Point | ICC 1 | 95% Confidence Intervals (Lower/Upper) | |
|---|---|---|---|
| T0 | 0.905 | 0.488 | 0.977 |
| T1 | 0.802 | 0.319 | 0.946 |
| T2 | 0.815 | 0.359 | 0.949 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gunasekara, N.; Clauss, D.; Voss, A.; Schurz, K.; Fleck, K.; Neu-Gil, P.; Bloch, W. The Influence of an Acute Endurance Intervention on Breast Cancer Cell Growth—A Pilot Study. Int. J. Mol. Sci. 2025, 26, 3976. https://doi.org/10.3390/ijms26093976
Gunasekara N, Clauss D, Voss A, Schurz K, Fleck K, Neu-Gil P, Bloch W. The Influence of an Acute Endurance Intervention on Breast Cancer Cell Growth—A Pilot Study. International Journal of Molecular Sciences. 2025; 26(9):3976. https://doi.org/10.3390/ijms26093976
Chicago/Turabian StyleGunasekara, Nadira, Dorothea Clauss, Anika Voss, Konstantin Schurz, Katharina Fleck, Pablo Neu-Gil, and Wilhelm Bloch. 2025. "The Influence of an Acute Endurance Intervention on Breast Cancer Cell Growth—A Pilot Study" International Journal of Molecular Sciences 26, no. 9: 3976. https://doi.org/10.3390/ijms26093976
APA StyleGunasekara, N., Clauss, D., Voss, A., Schurz, K., Fleck, K., Neu-Gil, P., & Bloch, W. (2025). The Influence of an Acute Endurance Intervention on Breast Cancer Cell Growth—A Pilot Study. International Journal of Molecular Sciences, 26(9), 3976. https://doi.org/10.3390/ijms26093976








