Therapeutic Potential of Local and Systemic Adipose-Derived Mesenchymal Stem Cell Injections in a Rat Model of Experimental Periodontitis: Implications for Cardiac Function
Abstract
1. Introduction
2. Results
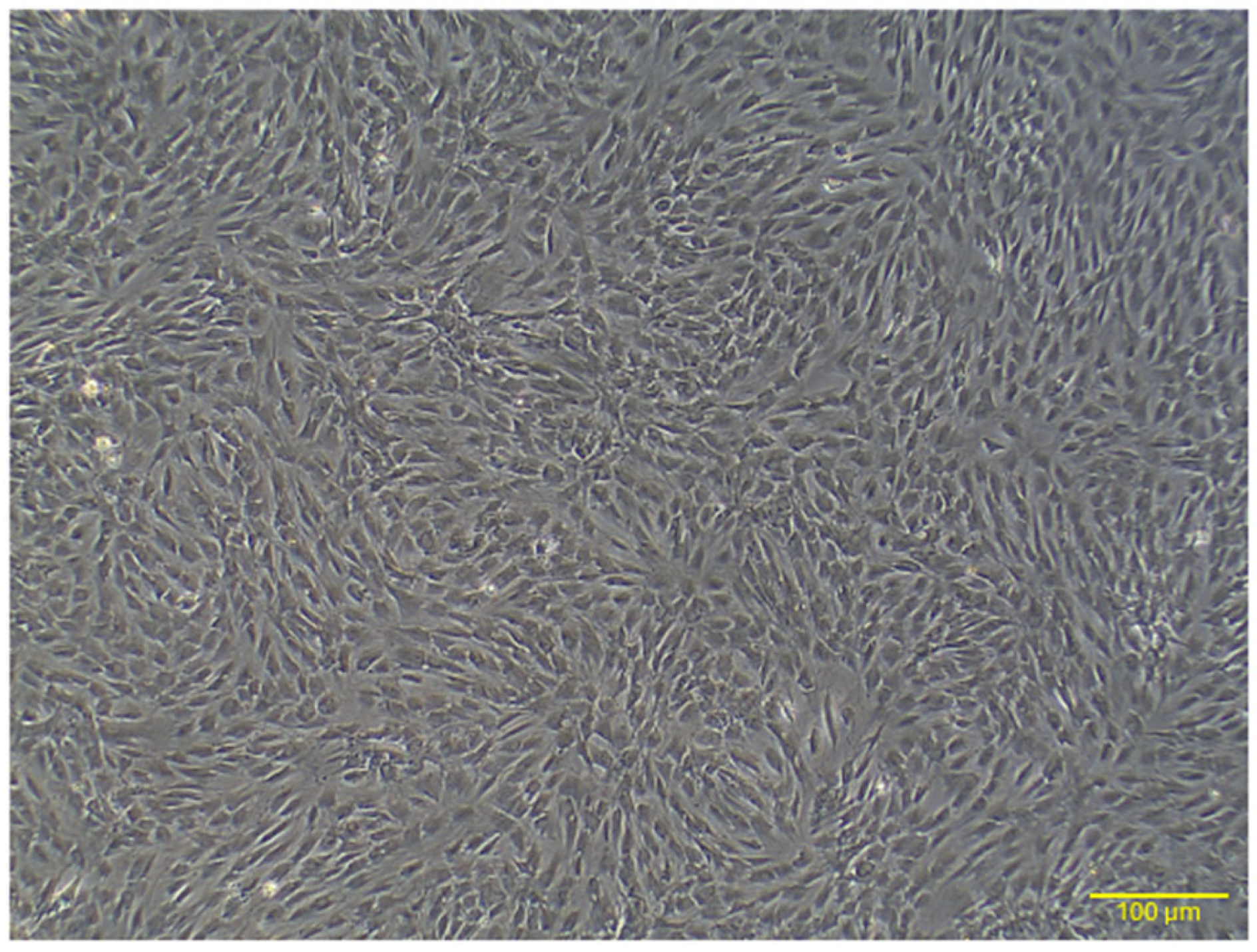
2.1. Characterization of Rat AD-MSCs
2.1.1. Morphology
2.1.2. Flow Cytometry Analysis
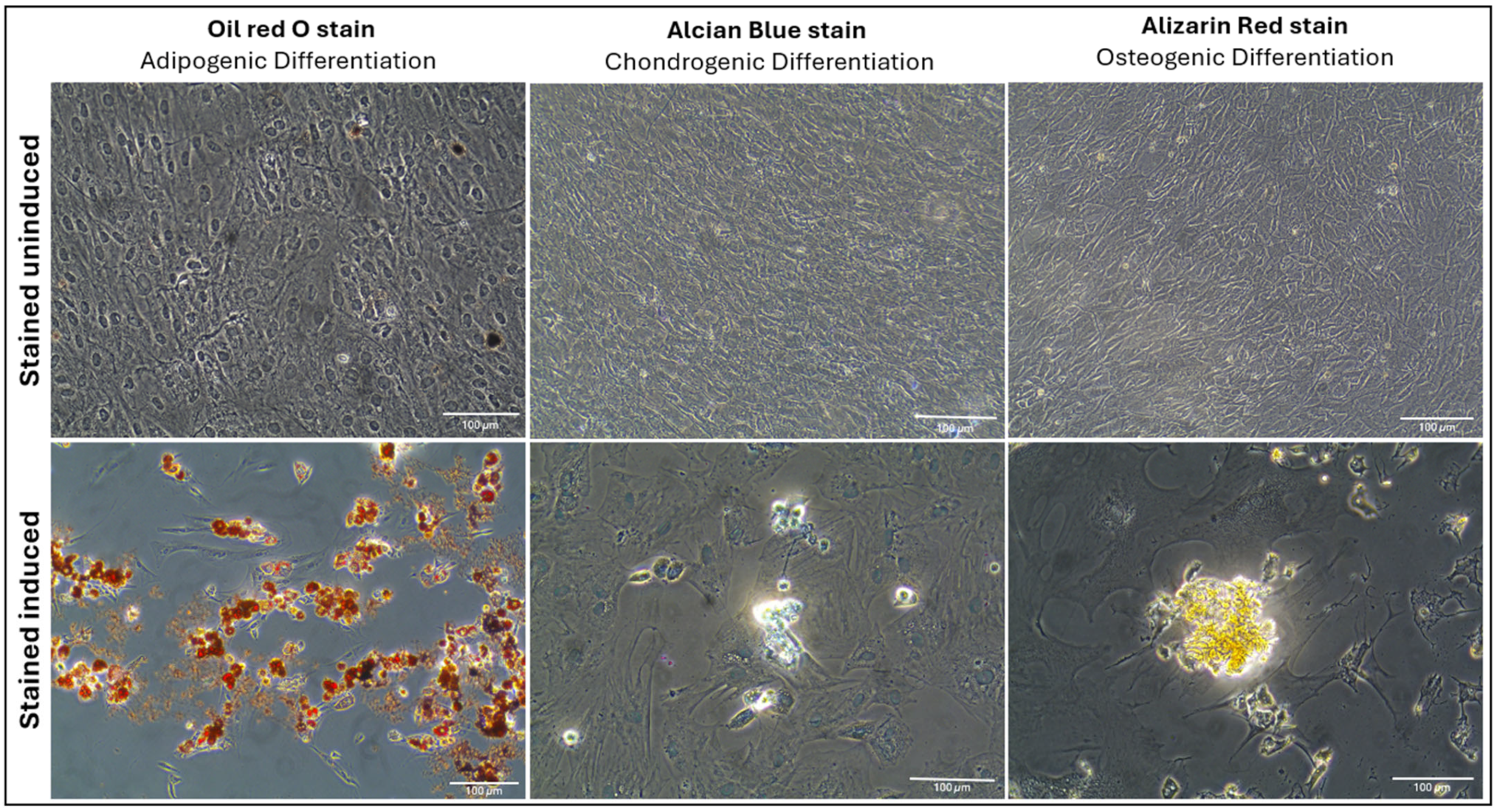
2.1.3. Multilineage Differentiation
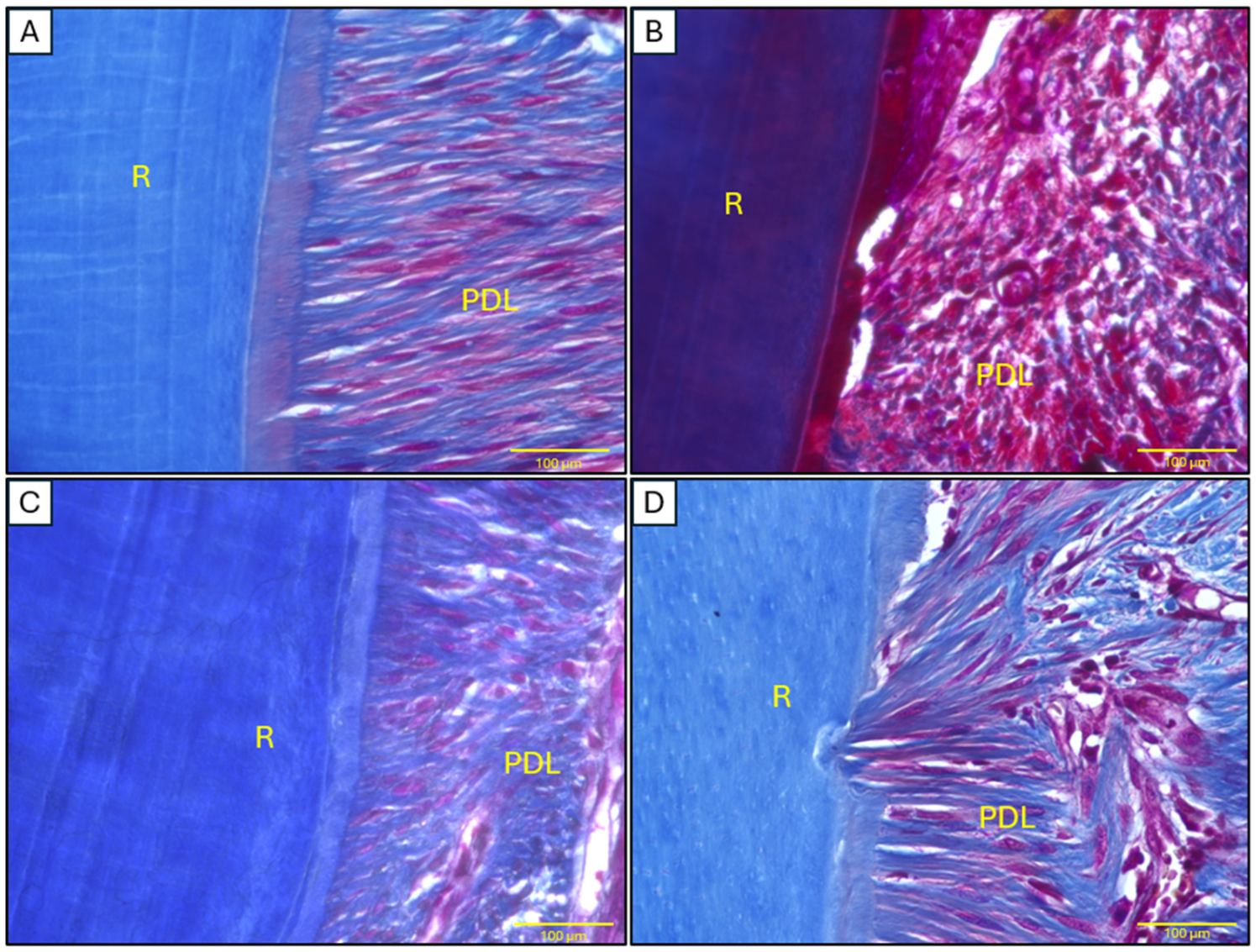
2.2. Bone Loss Assessment
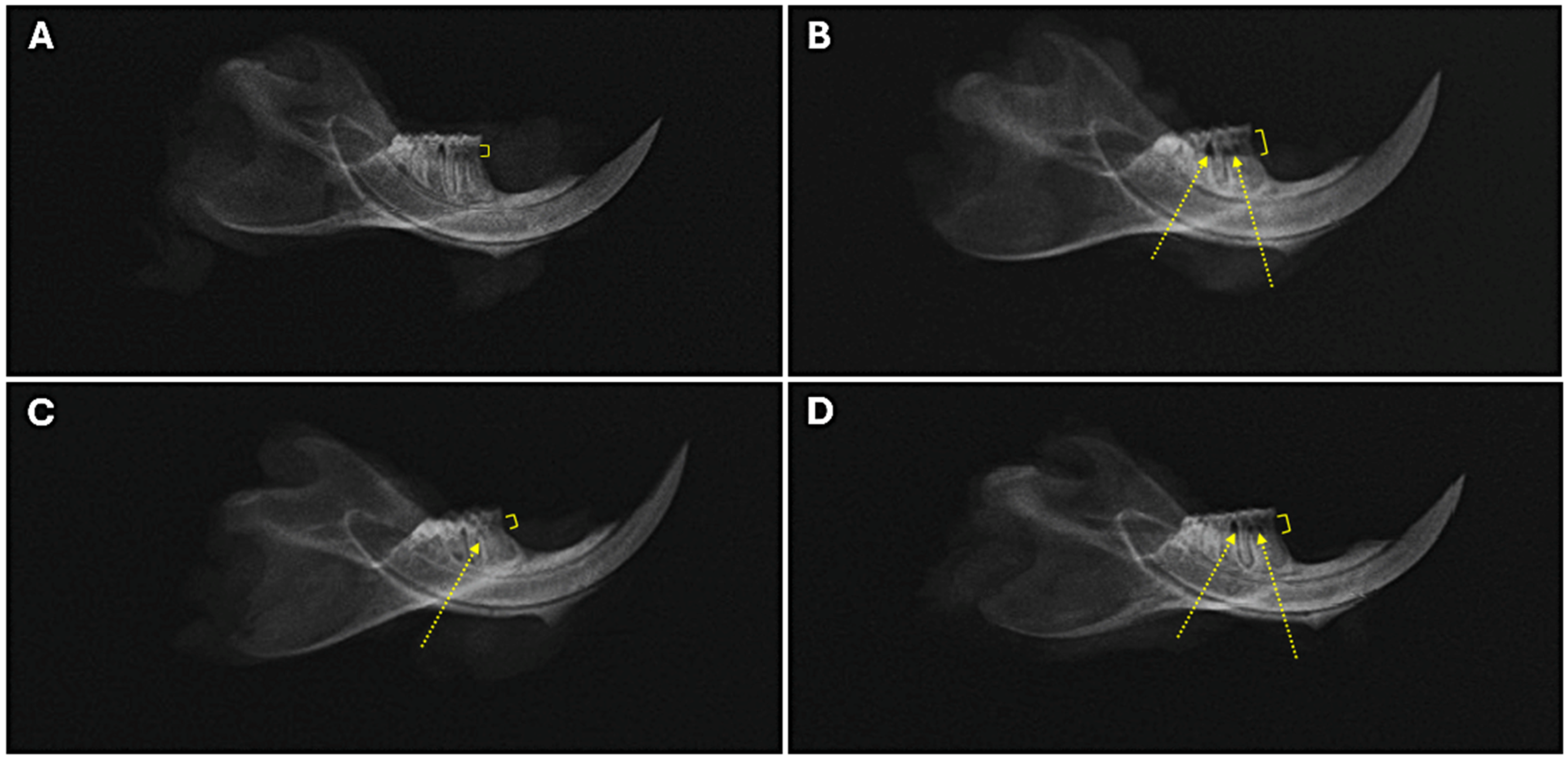
2.3. Alveolar Bone Radiographic Analyses
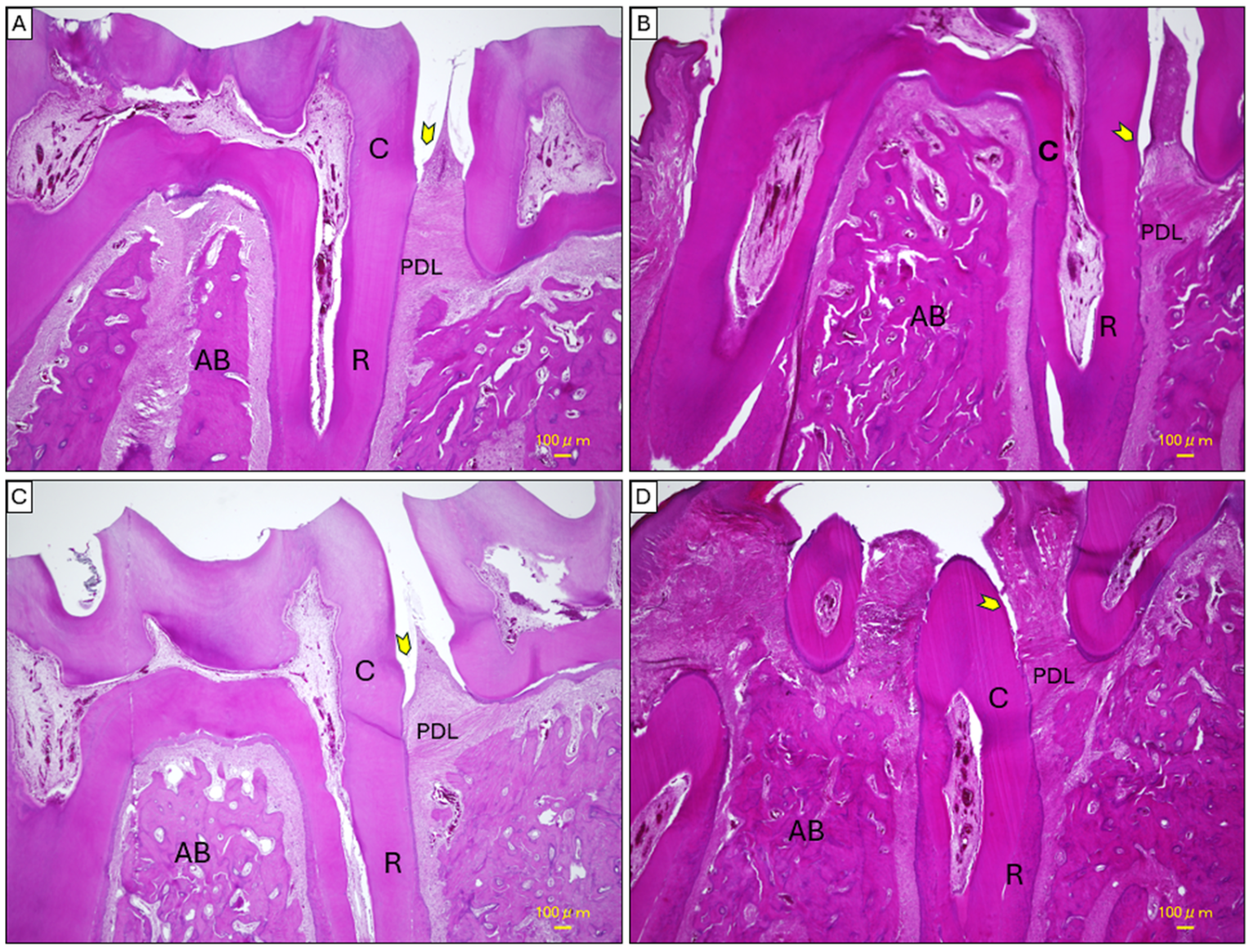
2.4. Histopathological Analysis of Mandibular Tissues
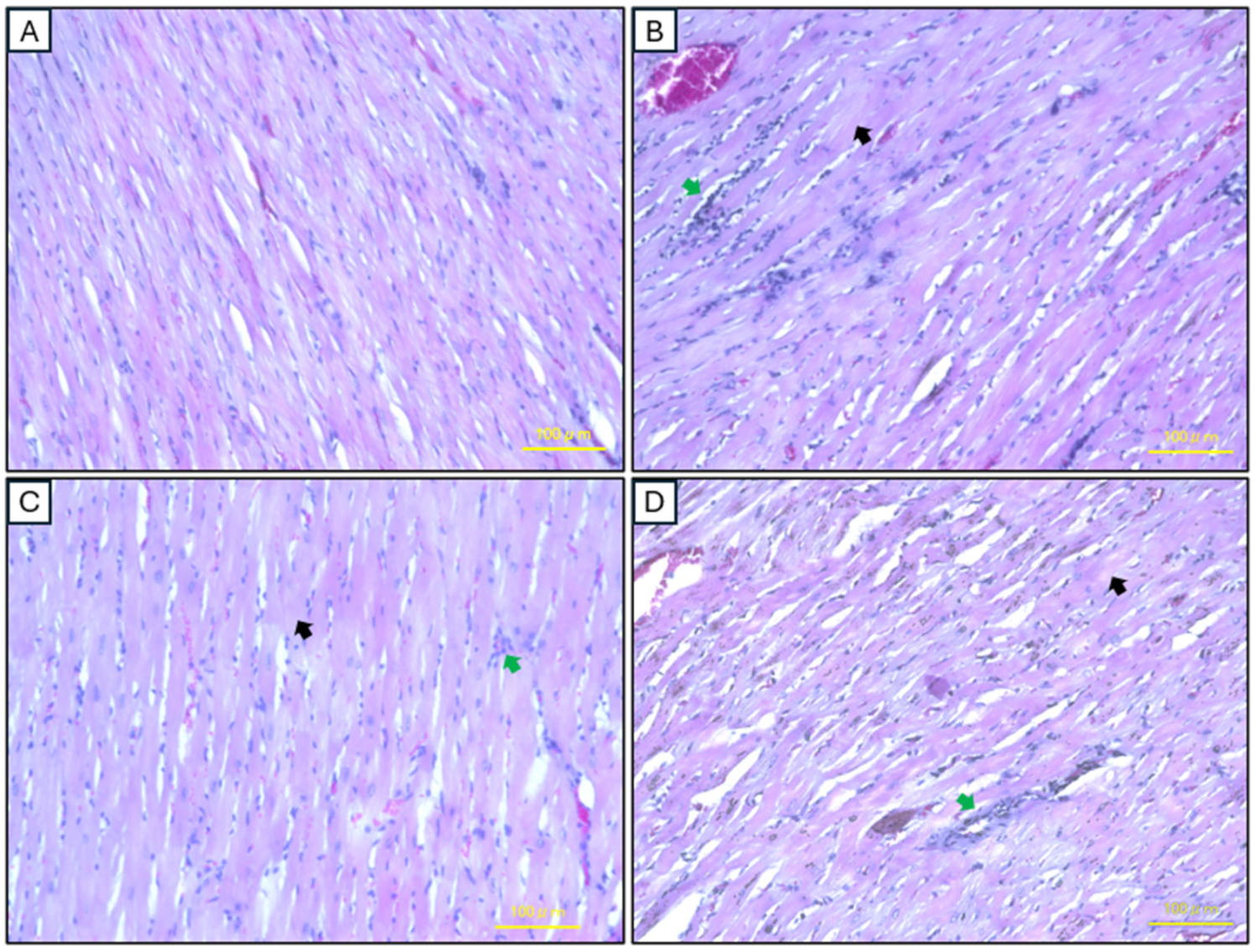
2.5. Histopathological Analysis of Cardiac Tissues
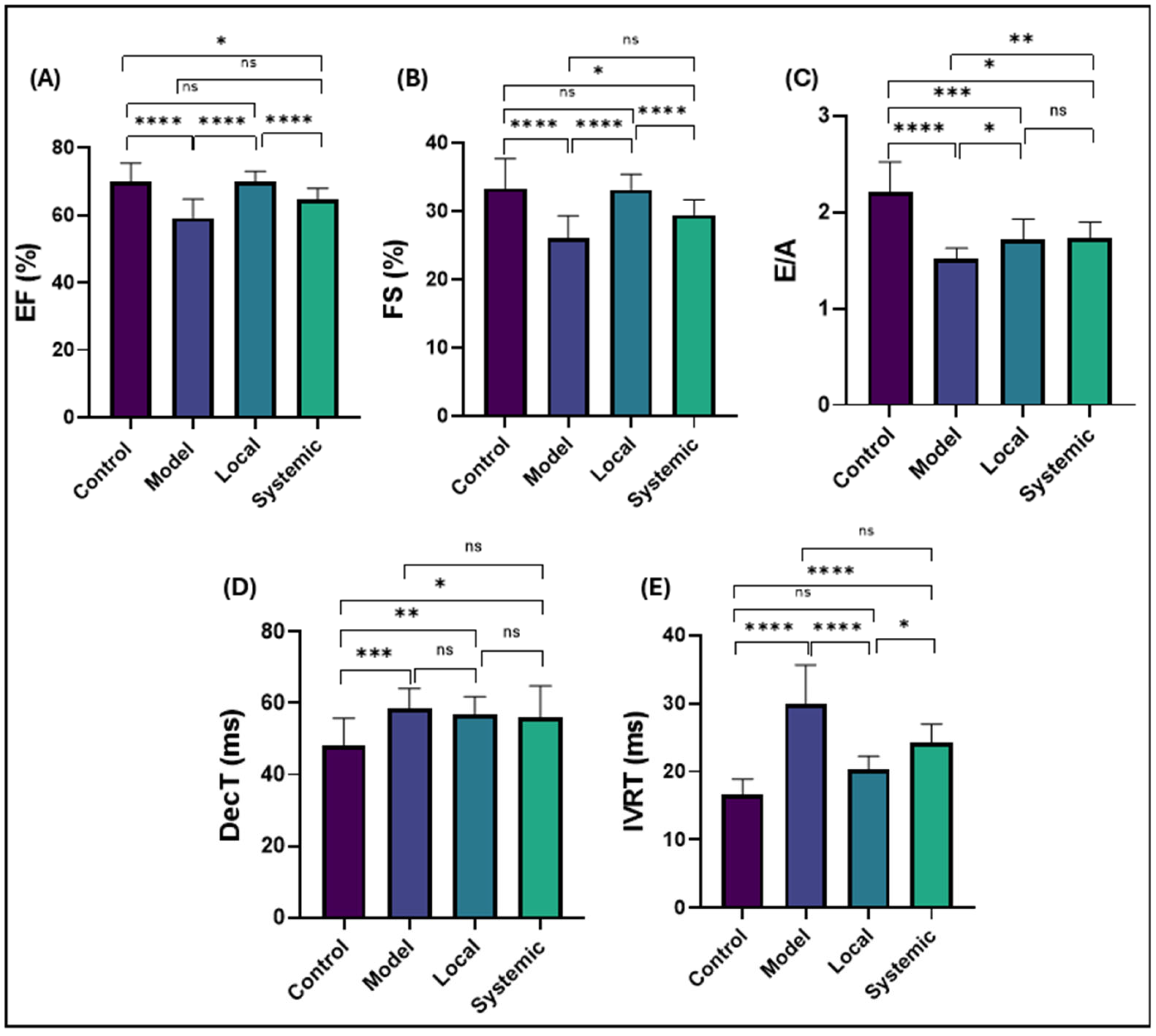
2.6. Conventional Echocardiographic Findings
3. Discussion
Clinical Significance of Findings
4. Materials and Methods
4.1. Animal Care and Ethical Compliance
4.2. Preparation and Culturing of AD-MSCs
4.3. Flow Cytometric Characterization of AD-MSCs
4.4. Multilineage Differentiation Assessment of AD-MSCs
4.4.1. Adipogenic Differentiation
4.4.2. Chondrogenic Differentiation
4.4.3. Osteogenic Differentiation
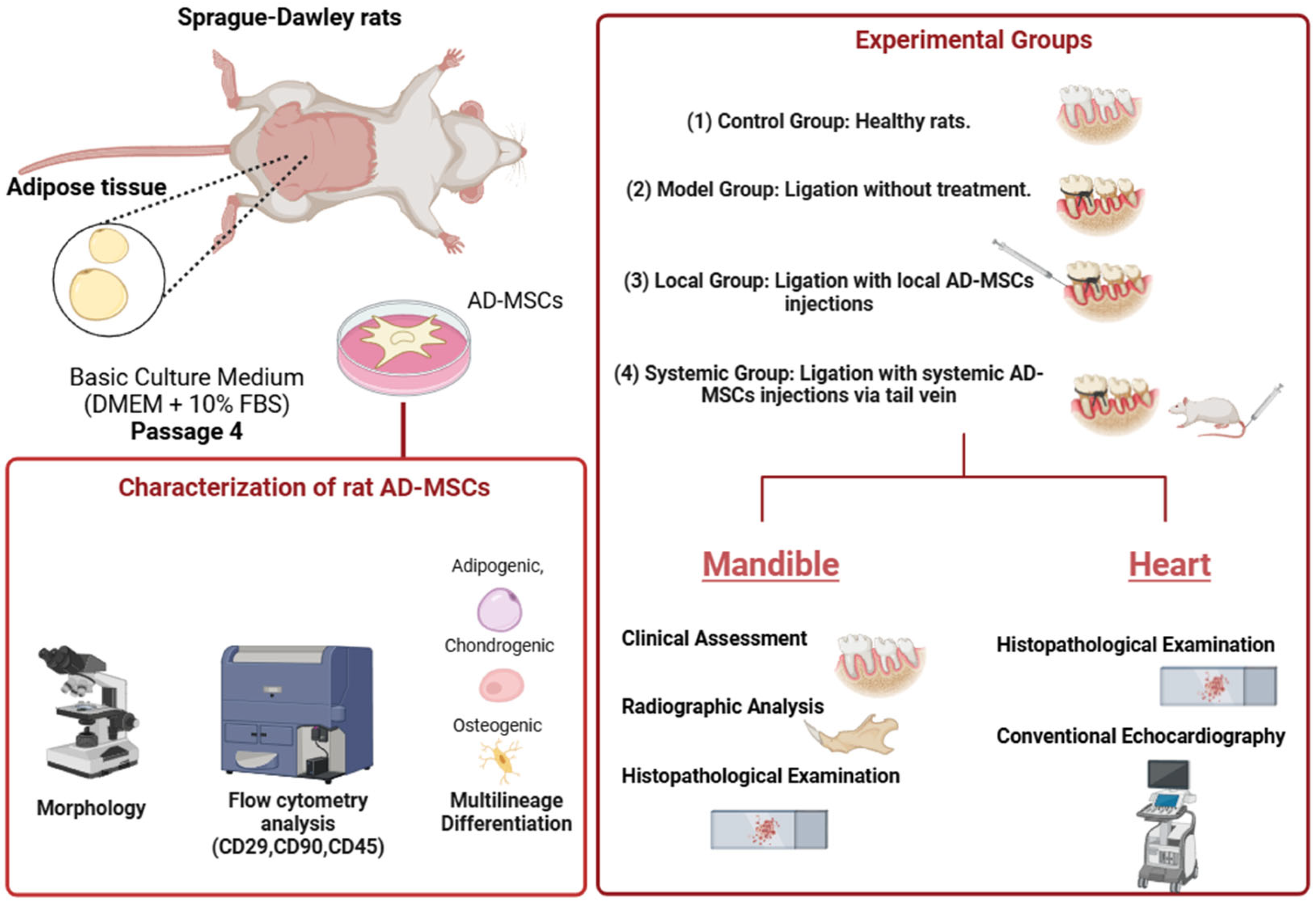
4.5. Experimental Groups and Treatment Protocols
- Control Group: No ligation or AD-MSC treatment.
- Model Group: Ligation-induced periodontitis without AD-MSC treatment.
- Local Group: Ligation followed by local AD-MSC injections (1 × 106 cells) delivered supraperiosteally near the bone surface.
- Systemic Group: Ligation followed by systemic AD-MSC injections (1 × 106 cells) via the tail vein.
4.6. Clinical Assessment of Periodontal Bone Loss
4.7. Radiographic Assessment of Alveolar Bone Loss and Periodontal Bone Support
4.8. Histopathological Examination
4.8.1. Mandibular Tissues
4.8.2. Cardiac Tissues
4.8.3. Staining Procedures
H&E Staining
Masson’s Trichrome Staining
4.9. Conventional Echocardiography
4.10. Statistical Analysis
5. Conclusions
Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pihlstrom, B.L.; Michalowicz, B.S.; Johnson, N.W. Periodontal Diseases. Lancet 2005, 366, 1809–1820. [Google Scholar] [CrossRef]
- Hynes, K.; Menicanin, D.; Gronthos, S.; Bartold, P.M. Clinical Utility of Stem Cells for Periodontal Regeneration. Periodontol. 2000 2012, 59, 203–227. [Google Scholar] [CrossRef]
- Lin, N.-H.; Gronthos, S.; Mark Bartold, P. Stem Cells and Future Periodontal Regeneration. Periodontol. 2000 2009, 51, 239–251. [Google Scholar] [CrossRef]
- Chen, F.-M.; Jin, Y. Periodontal Tissue Engineering and Regeneration: Current Approaches and Expanding Opportunities. Tissue Eng. Part B Rev. 2010, 16, 219–255. [Google Scholar] [CrossRef]
- Sanz, M.; Ceriello, A.; Buysschaert, M.; Chapple, I.; Demmer, R.T.; Graziani, F.; Herrera, D.; Jepsen, S.; Lione, L.; Madianos, P. Scientific Evidence on the Links between Periodontal Diseases and Diabetes: Consensus Report and Guidelines of the Joint Workshop on Periodontal Diseases and Diabetes by the International Diabetes Federation and the European Federation of Periodontology. Diabetes Res. Clin. Pract. 2018, 137, 231–241. [Google Scholar] [CrossRef]
- Olsen, I.; Yilmaz, Ö. Modulation of Inflammasome Activity by Porphyromonas Gingivalis in Periodontitis and Associated Systemic Diseases. J. Oral Microbiol. 2016, 8, 30385. [Google Scholar] [CrossRef]
- Ruan, Q.; Guan, P.; Qi, W.; Li, J.; Xi, M.; Xiao, L.; Zhong, S.; Ma, D.; Ni, J. Porphyromonas Gingivalis Regulates Atherosclerosis through an Immune Pathway. Front. Immunol. 2023, 14, 1103592. [Google Scholar] [CrossRef]
- Kozarov, E.V.; Dorn, B.R.; Shelburne, C.E.; Dunn, W.A., Jr.; Progulske-Fox, A. Human Atherosclerotic Plaque Contains Viable Invasive Actinobacillus Actinomycetemcomitans and Porphyromonas Gingivalis. Arterioscler. Thromb. Vasc. Biol. 2005, 25, e17–e18. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, J.; Ouyang, X.; Lyu, P.; Wang, Y.; Zhong, J. Modulation of Lectin-like Oxidized Low-Density Lipoprotein Receptor-1 by Porphyromonas Gingivalis Promoting Progression of Atherosclerosis in Apolipoprotein E-/-Mice. J. Dent. Sci. 2025, 20, 754–763. [Google Scholar] [CrossRef]
- Yu, G.; Yu, Y.; Li, Y.N.; Shu, R. Effect of Periodontitis on Susceptibility to Atrial Fibrillation in an Animal Model. J. Electrocardiol. 2010, 43, 359–366. [Google Scholar] [CrossRef]
- Doughan, M.; Chehab, O.; de Vasconcellos, H.D.; Zeitoun, R.; Varadarajan, V.; Doughan, B.; Wu, C.O.; Blaha, M.J.; Bluemke, D.A.; Lima, J.A.C. Periodontal Disease Associated with Interstitial Myocardial Fibrosis: The Multiethnic Study of Atherosclerosis. J. Am. Heart Assoc. 2023, 12, e8146. [Google Scholar] [CrossRef] [PubMed]
- Grundtman, C.; Kreutmayer, S.B.; Almanzar, G.; Wick, M.C.; Wick, G. Heat Shock Protein 60 and Immune Inflammatory Responses in Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 960–968. [Google Scholar] [CrossRef] [PubMed]
- Díaz, C.M.; Bullon, B.; Ruiz-Salmerón, R.J.; Fernández-Riejos, P.; Fernández-Palacín, A.; Battino, M.; Cordero, M.D.; Quiles, J.L.; Varela-López, A.; Bullón, P. Molecular Inflammation and Oxidative Stress Are Shared Mechanisms Involved in Both Myocardial Infarction and Periodontitis. J. Periodontal Res. 2020, 55, 519–528. [Google Scholar] [CrossRef]
- Caplan, A.I. Mesenchymal Stem Cells. J. Orthop. Res. 1991, 9, 641–650. [Google Scholar] [CrossRef]
- Friedenstein, A.J. Precursor Cells of Mechanocytes. Int. Rev. Cytol. 1976, 47, 327–359. [Google Scholar]
- Dennis, J.E.; Merriam, A.; Awadallah, A.; Yoo, J.U.; Johnstone, B.; Caplan, A.I. A Quadripotential Mesenchymal Progenitor Cell Isolated from the Marrow of an Adult Mouse. J. Bone Miner. Res. 1999, 14, 700–709. [Google Scholar] [CrossRef] [PubMed]
- Vilquin, J.-T.; Rosset, P. Mesenchymal Stem Cells in Bone and Cartilage Repair: Current Status. Regen. Med. 2006, 1, 589–604. [Google Scholar] [CrossRef]
- Veronesi, F.; Giavaresi, G.; Tschon, M.; Borsari, V.; Nicoli Aldini, N.; Fini, M. Clinical Use of Bone Marrow, Bone Marrow Concentrate, and Expanded Bone Marrow Mesenchymal Stem Cells in Cartilage Disease. Stem Cells Dev. 2013, 22, 181–192. [Google Scholar] [CrossRef]
- Caplan, A.I.; Dennis, J.E. Mesenchymal Stem Cells as Trophic Mediators. J. Cell. Biochem. 2006, 98, 1076–1084. [Google Scholar] [CrossRef]
- Farag, A.; Hendawy, H.; Emam, M.H.; Hasegawa, M.; Mandour, A.S.; Tanaka, R. Stem Cell Therapies in Canine Cardiology: Comparative Efficacy, Emerging Trends, and Clinical Integration. Biomolecules 2025, 15, 371. [Google Scholar] [CrossRef]
- Kean, T.J.; Lin, P.; Caplan, A.I.; Dennis, J.E. MSCs: Delivery Routes and Engraftment, Cell-targeting Strategies, and Immune Modulation. Stem Cells Int. 2013, 2013, 732742. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Liu, Y.; Zhang, X.; Lin, J. The Therapeutic Role of Bone Marrow Stem Cell Local Injection in Rat Experimental Periodontitis. J. Oral Rehabil. 2020, 47, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Mandl, S.; Schimmelpfennig, C.; Edinger, M.; Negrin, R.S.; Contag, C.H. Understanding Immune Cell Trafficking Patterns via in Vivo Bioluminescence Imaging. J. Cell. Biochem. 2002, 87, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Cao, F.; Lin, S.; Xie, X.; Ray, P.; Patel, M.; Zhang, X.; Drukker, M.; Dylla, S.J.; Connolly, A.J.; Chen, X. In Vivo Visualization of Embryonic Stem Cell Survival, Proliferation, and Migration after Cardiac Delivery. Circulation 2006, 113, 1005–1014. [Google Scholar] [CrossRef]
- Wang, X.; Rosol, M.; Ge, S.; Peterson, D.; McNamara, G.; Pollack, H.; Kohn, D.B.; Nelson, M.D.; Crooks, G.M. Dynamic Tracking of Human Hematopoietic Stem Cell Engraftment Using in Vivo Bioluminescence Imaging. Blood 2003, 102, 3478–3482. [Google Scholar] [CrossRef]
- Caton, J.G.; Armitage, G.; Berglundh, T.; Chapple, I.L.C.; Jepsen, S.; Kornman, K.S.; Mealey, B.L.; Papapanou, P.N.; Sanz, M.; Tonetti, M.S. A New Classification Scheme for Periodontal and Peri-implant Diseases and Conditions–Introduction and Key Changes from the 1999 Classification. J. Periodontol. 2018, 89, S1–S8. [Google Scholar] [CrossRef]
- Rojas, M.A.; Marini, L.; Pilloni, A.; Sahrmann, P. Early wound healing outcomes after regenerative periodontal surgery with enamel matrix derivatives or guided tissue regeneration: A systematic review. BMC Oral Health 2019, 19, 1–16. [Google Scholar] [CrossRef]
- Chen, F.-M.; Gao, L.-N.; Tian, B.-M.; Zhang, X.-Y.; Zhang, Y.-J.; Dong, G.-Y.; Lu, H.; Chu, Q.; Xu, J.; Yu, Y. Treatment of Periodontal Intrabony Defects Using Autologous Periodontal Ligament Stem Cells: A Randomized Clinical Trial. Stem Cell Res. Ther. 2016, 7, 33. [Google Scholar] [CrossRef]
- Ferrarotti, F.; Romano, F.; Gamba, M.N.; Quirico, A.; Giraudi, M.; Audagna, M.; Aimetti, M. Human Intrabony Defect Regeneration with Micrografts Containing Dental Pulp Stem Cells: A Randomized Controlled Clinical Trial. J. Clin. Periodontol. 2018, 45, 841–850. [Google Scholar] [CrossRef]
- Behnia, H.; Khojasteh, A.; Soleimani, M.; Tehranchi, A.; Khoshzaban, A.; Keshel, S.H.; Atashi, R. Secondary Repair of Alveolar Clefts Using Human Mesenchymal Stem Cells. Oral Surgery Oral Med. Oral Pathol. Oral Radiol. Endodontology 2009, 108, e1–e6. [Google Scholar] [CrossRef]
- Lalu, M.M.; Mazzarello, S.; Zlepnig, J.; Dong, Y.Y.; Montroy, J.; McIntyre, L.; Devereaux, P.J.; Stewart, D.J.; David Mazer, C.; Barron, C.C. Safety and Efficacy of Adult Stem Cell Therapy for Acute Myocardial Infarction and Ischemic Heart Failure (SafeCell Heart): A Systematic Review and Meta-Analysis. Stem Cells Transl. Med. 2018, 7, 857–866. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Mu, H.; Shen, Z.; Song, Z.; Chen, X.; Wang, Y. Autologous Adipose Tissue-derived Mesenchymal Stem Cells Are Involved in Rat Liver Regeneration Following Repeat Partial Hepatectomy. Mol. Med. Rep. 2016, 13, 2053–2059. [Google Scholar] [CrossRef] [PubMed]
- Gong, M.; Bi, Y.; Jiang, W.; Zhang, Y.; Chen, L.; Hou, N.; Liu, Y.; Wei, X.; Chen, J.; Li, T. Immortalized Mesenchymal Stem Cells: An Alternative to Primary Mesenchymal Stem Cells in Neuronal Differentiation and Neuroregeneration Associated Studies. J. Biomed. Sci. 2011, 18, 87. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage Potential of Adult Human Mesenchymal Stem Cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal Criteria for Defining Multipotent Mesenchymal Stromal Cells. The International Society for Cellular Therapy Position Statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Kemp, K.; Morse, R.; Wexler, S.; Cox, C.; Mallam, E.; Hows, J.; Donaldson, C. Chemotherapy-Induced Mesenchymal Stem Cell Damage in Patients with Hematological Malignancy. Ann. Hematol. 2010, 89, 701–713. [Google Scholar] [CrossRef]
- Ke, C.; Chen, J.; Guo, Y.; Chen, Z.W.; Cai, J. Migration Mechanism of Mesenchymal Stem Cells Studied by QD/NSOM. Biochim. Biophys. Acta (BBA)-Biomembr. 2015, 1848, 859–868. [Google Scholar] [CrossRef]
- Chamberlain, G.; Fox, J.; Ashton, B.; Middleton, J. Concise Review: Mesenchymal Stem Cells: Their Phenotype, Differentiation Capacity, Immunological Features, and Potential for Homing. Stem Cells 2007, 25, 2739–2749. [Google Scholar] [CrossRef]
- Hendawy, H.; Kaneda, M.; Yoshida, T.; Metwally, E.; Hamabe, L.; Yoshida, T.; Shimada, K.; Tanaka, R. Heterogeneity of Adipose Stromal Vascular Fraction Cells from the Different Harvesting Sites in Rats. Anat. Rec. 2022, 305, 3410–3421. [Google Scholar] [CrossRef]
- Hendawy, H.; Kaneda, M.; Metwally, E.; Shimada, K.; Tanaka, T.; Tanaka, R. A Comparative Study of the Effect of Anatomical Site on Multiple Differentiation of Adipose-Derived Stem Cells in Rats. Cells 2021, 10, 2469. [Google Scholar] [CrossRef]
- Farag, A.; Koung Ngeun, S.; Kaneda, M.; Aboubakr, M.; Tanaka, R. Optimizing Cardiomyocyte Differentiation: Comparative Analysis of Bone Marrow and Adipose-Derived Mesenchymal Stem Cells in Rats Using 5-Azacytidine and Low-Dose FGF and IGF Treatment. Biomedicines 2024, 12, 1923. [Google Scholar] [CrossRef] [PubMed]
- Farag, A.; Ngeun, S.K.; Kaneda, M.; Aboubakr, M.; Elhaieg, A.; Hendawy, H.; Tanaka, R. Exploring the Potential Effects of Cryopreservation on the Biological Characteristics and Cardiomyogenic Differentiation of Rat Adipose-Derived Mesenchymal Stem Cells. Int. J. Mol. Sci. 2024, 25, 9908. [Google Scholar] [CrossRef] [PubMed]
- Koung Ngeun, S.; Shimizu, M.; Kaneda, M. Myogenic Differentiation and Immunomodulatory Properties of Rat Adipose-Derived Mesenchymal Stem/Stromal Cells. Biology 2024, 13, 72. [Google Scholar] [CrossRef]
- Melief, S.M.; Zwaginga, J.J.; Fibbe, W.E.; Roelofs, H. Adipose Tissue-Derived Multipotent Stromal Cells Have a Higher Immunomodulatory Capacity than Their Bone Marrow-Derived Counterparts. Stem Cells Transl. Med. 2013, 2, 455–463. [Google Scholar] [CrossRef]
- Tobita, M.; Orbay, H.; Mizuno, H. Adipose-Derived Stem Cells: Current Findings and Future Perspectives. Discov. Med. 2011, 11, 160–170. [Google Scholar]
- Zuk, P.A.; Zhu, M.I.N.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multilineage Cells from Human Adipose Tissue: Implications for Cell-Based Therapies. Tissue Eng. 2001, 7, 211–228. [Google Scholar] [CrossRef]
- Della Rocca, Y.; Diomede, F.; Konstantinidou, F.; Trubiani, O.; Soundara Rajan, T.; Pierdomenico, S.D.; Gatta, V.; Stuppia, L.; Marconi, G.D.; Pizzicannella, J. Protective Effect of Oral Stem Cells Extracellular Vesicles on Cardiomyocytes in Hypoxia-Reperfusion. Front. Cell Dev. Biol. 2024, 11, 1260019. [Google Scholar] [CrossRef]
- Della Rocca, Y.; Diomede, F.; Konstantinidou, F.; Gatta, V.; Stuppia, L.; Benedetto, U.; Zimarino, M.; Lanuti, P.; Trubiani, O.; Pizzicannella, J. Autologous HGMSC-Derived IPS: A New Proposal for Tissue Regeneration. Int. J. Mol. Sci. 2024, 25, 9169. [Google Scholar] [CrossRef]
- Du, J.; Shan, Z.; Ma, P.; Wang, S.; Fan, Z. Allogeneic Bone Marrow Mesenchymal Stem Cell Transplantation for Periodontal Regeneration. J. Dent. Res. 2014, 93, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Zhu, D.; Fan, Y.; Liu, H.; Shen, Z. Human Umbilical Cord Mesenchymal Stem Cells-Derived Extracellular Vesicles for Rat Jawbone Regeneration in Periapical Periodontitis. ACS Biomater. Sci. Eng. 2024, 10, 5784–5795. [Google Scholar] [CrossRef]
- Pawitan, J.A. Prospect of Stem Cell Conditioned Medium in Regenerative Medicine. Biomed Res. Int. 2014, 2014, 965849. [Google Scholar] [CrossRef]
- Inukai, T.; Katagiri, W.; Yoshimi, R.; Osugi, M.; Kawai, T.; Hibi, H.; Ueda, M. Novel Application of Stem Cell-Derived Factors for Periodontal Regeneration. Biochem. Biophys. Res. Commun. 2013, 430, 763–768. [Google Scholar] [CrossRef] [PubMed]
- Madrigal, M.; Rao, K.S.; Riordan, N.H. A Review of Therapeutic Effects of Mesenchymal Stem Cell Secretions and Induction of Secretory Modification by Different Culture Methods. J. Transl. Med. 2014, 12, 260. [Google Scholar] [CrossRef] [PubMed]
- Ratajczak, M.Z.; Kucia, M.; Jadczyk, T.; Greco, N.J.; Wojakowski, W.; Tendera, M.; Ratajczak, J. Pivotal Role of Paracrine Effects in Stem Cell Therapies in Regenerative Medicine: Can We Translate Stem Cell-Secreted Paracrine Factors and Microvesicles into Better Therapeutic Strategies? Leukemia 2012, 26, 1166–1173. [Google Scholar] [CrossRef]
- Kyurkchiev, D.; Bochev, I.; Ivanova-Todorova, E.; Mourdjeva, M.; Oreshkova, T.; Belemezova, K.; Kyurkchiev, S. Secretion of Immunoregulatory Cytokines by Mesenchymal Stem Cells. World J. Stem Cells 2014, 6, 552. [Google Scholar] [CrossRef]
- Wu, Y.; Zhao, R.C.H. The Role of Chemokines in Mesenchymal Stem Cell Homing to Myocardium. Stem Cell Rev. Rep. 2012, 8, 243–250. [Google Scholar] [CrossRef]
- Li, S.; Tu, Q.; Zhang, J.; Stein, G.; Lian, J.; Yang, P.S.; Chen, J. Systemically Transplanted Bone Marrow Stromal Cells Contributing to Bone Tissue Regeneration. J. Cell. Physiol. 2008, 215, 204–209. [Google Scholar] [CrossRef]
- Jackson, J.S.; Golding, J.P.; Chapon, C.; Jones, W.A.; Bhakoo, K.K. Homing of Stem Cells to Sites of Inflammatory Brain Injury after Intracerebral and Intravenous Administration: A Longitudinal Imaging Study. Stem Cell Res. Ther. 2010, 1, 17. [Google Scholar] [CrossRef]
- Ortiz, L.A.; Gambelli, F.; McBride, C.; Gaupp, D.; Baddoo, M.; Kaminski, N.; Phinney, D.G. Mesenchymal Stem Cell Engraftment in Lung Is Enhanced in Response to Bleomycin Exposure and Ameliorates Its Fibrotic Effects. Proc. Natl. Acad. Sci. USA 2003, 100, 8407–8411. [Google Scholar] [CrossRef]
- Xu, Q.-C.; Hao, P.-J.; Yu, X.-B.; Chen, S.-L.; Yu, M.-J.; Zhang, J.; Yang, P.-S. Hyperlipidemia Compromises Homing Efficiency of Systemically Transplanted BMSCs and Inhibits Bone Regeneration. Int. J. Clin. Exp. Pathol. 2014, 7, 1580. [Google Scholar]
- Wang, X.; Chen, J.; Tian, W. Strategies of Cell and Cell-Free Therapies for Periodontal Regeneration: The State of the Art. Stem Cell Res. Ther. 2022, 13, 536. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, H.; Hirachi, A.; Hasegawa, N.; Iwata, T.; Hamaguchi, H.; Shiba, H.; Takata, T.; Kato, Y.; Kurihara, H. Enhancement of Periodontal Tissue Regeneration by Transplantation of Bone Marrow Mesenchymal Stem Cells. J. Periodontol. 2004, 75, 1281–1287. [Google Scholar] [CrossRef]
- Fischer, U.M.; Harting, M.T.; Jimenez, F.; Monzon-Posadas, W.O.; Xue, H.; Savitz, S.I.; Laine, G.A.; Cox, C.S., Jr. Pulmonary Passage Is a Major Obstacle for Intravenous Stem Cell Delivery: The Pulmonary First-Pass Effect. Stem Cells Dev. 2009, 18, 683–692. [Google Scholar] [CrossRef]
- Terrizzi, A.R.; Fernandez-Solari, J.; Lee, C.M.; Bozzini, C.; Mandalunis, P.M.; Elverdin, J.C.; Conti, M.I.; Martínez, M.P. Alveolar Bone Loss Associated to Periodontal Disease in Lead Intoxicated Rats under Environmental Hypoxia. Arch. Oral Biol. 2013, 58, 1407–1414. [Google Scholar] [CrossRef]
- Ionel, A.; Lucaciu, O.; Moga, M.; Buhatel, D.; Ilea, A.; Tabaran, F.; Catoi, C.; Berce, C.; Toader, S.; Campian, R.S. Periodontal Disease Induced in Wistar Rats-Experimental Study. Hum. Vet. Med. 2015, 7, 90–95. [Google Scholar]
- Li, G.; Han, N.; Zhang, X.; Yang, H.; Cao, Y.; Wang, S.; Fan, Z. Local Injection of Allogeneic Stem Cells from Apical Papilla Enhanced Periodontal Tissue Regeneration in Minipig Model of Periodontitis. Biomed Res. Int. 2018, 2018, 3960798. [Google Scholar] [CrossRef]
- Morsczeck, C.; Schmalz, G.; Reichert, T.E.; Völlner, F.; Galler, K.; Driemel, O. Somatic Stem Cells for Regenerative Dentistry. Clin. Oral Investig. 2008, 12, 113–118. [Google Scholar] [CrossRef]
- Mohammed, E.; Khalil, E.; Sabry, D. Effect of Adipose-Derived Stem Cells and Their Exo as Adjunctive Therapy to Nonsurgical Periodontal Treatment: A Histologic and Histomorphometric Study in Rats. Biomolecules 2018, 8, 167. [Google Scholar] [CrossRef]
- Bassir, S.H.; Wisitrasameewong, W.; Raanan, J.; Ghaffarigarakani, S.; Chung, J.; Freire, M.; Andrada, L.C.; Intini, G. Potential for Stem Cell-based Periodontal Therapy. J. Cell. Physiol. 2016, 231, 50–61. [Google Scholar] [CrossRef]
- Lemaitre, M.; Monsarrat, P.; Blasco-Baque, V.; Loubières, P.; Burcelin, R.; Casteilla, L.; Planat-Bénard, V.; Kémoun, P. Periodontal Tissue Regeneration Using Syngeneic Adipose-Derived Stromal Cells in a Mouse Model. Stem Cells Transl. Med. 2017, 6, 656–665. [Google Scholar] [CrossRef]
- Salgado, A.J.; Reis, R.L.; Sousa, N.; Gimble, J.M. Adipose Tissue Derived Stem Cells Secretome: Soluble Factors and Their Roles in Regenerative Medicine. Curr. Stem Cell Res. Ther. 2010, 5, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Kim, H.K.; Cho, H.H.; Bae, Y.C.; Suh, K.T.; Jung, J.S. Direct Comparison of Human Mesenchymal Stem Cells Derived from Adipose Tissues and Bone Marrow in Mediating Neovascularization in Response to Vascular Ischemia. Cell. Physiol. Biochem. 2007, 20, 867–876. [Google Scholar] [CrossRef] [PubMed]
- Rehman, J.; Traktuev, D.; Li, J.; Merfeld-Clauss, S.; Temm-Grove, C.J.; Bovenkerk, J.E.; Pell, C.L.; Johnstone, B.H.; Considine, R.V.; March, K.L. Secretion of Angiogenic and Antiapoptotic Factors by Human Adipose Stromal Cells. Circulation 2004, 109, 1292–1298. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Wang, Z.; Xu, Q.; Sun, H.; Liu, X.; Yang, J.; Hong, R. The Treatment of Systematically Transplanted Gingival Mesenchymal Stem Cells in Periodontitis in Mice. Exp. Ther. Med. 2019, 17, 2199–2205. [Google Scholar] [CrossRef]
- Xu, Q.-C.; Wang, Z.-G.; Ji, Q.-X.; Yu, X.-B.; Xu, X.-Y.; Yuan, C.-Q.; Deng, J.; Yang, P.-S. Systemically Transplanted Human Gingiva-Derived Mesenchymal Stem Cells Contributing to Bone Tissue Regeneration. Int. J. Clin. Exp. Pathol. 2014, 7, 4922. [Google Scholar]
- Chen, F.-M.; Sun, H.-H.; Lu, H.; Yu, Q. Stem Cell-Delivery Therapeutics for Periodontal Tissue Regeneration. Biomaterials 2012, 33, 6320–6344. [Google Scholar] [CrossRef]
- Farag, A.; Mandour, A.S.; Kaneda, M.; Elfadadny, A.; Elhaieg, A.; Shimada, K.; Tanaka, R. Effect of Trehalose on Heart Functions in Rats Model after Myocardial Infarction: Assessment of Novel Intraventricular Pressure and Heart Rate Variability. Front. Cardiovasc. Med. 2023, 10, 1182628. [Google Scholar] [CrossRef]
- Farag, A.; Elfadadny, A.; Mandour, A.S.; Ngeun, S.K.; Aboubakr, M.; Kaneda, M.; Tanaka, R. Potential Protective Effects of L-Carnitine against Myocardial Ischemia/Reperfusion Injury in a Rat Model. Environ. Sci. Pollut. Res. 2024, 31, 18813–18825. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, X.; Lu, B.; Zhang, C.; Cai, Z. Can Apical Periodontitis Affect Serum Levels of CRP, IL-2, and IL-6 as Well as Induce Pathological Changes in Remote Organs? Clin. Oral Investig. 2016, 20, 1617–1624. [Google Scholar] [CrossRef]
- Dye, B.A.; Choudhary, K.; Shea, S.; Papapanou, P.N. Serum Antibodies to Periodontal Pathogens and Markers of Systemic Inflammation. J. Clin. Periodontol. 2005, 32, 1189–1199. [Google Scholar] [CrossRef]
- Stöllberger, C.; Finsterer, J. Role of Infectious and Immune Factors in Coronary and Cerebrovascular Arteriosclerosis. Clin. Vaccine Immunol. 2002, 9, 207–215. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sehirli, A.Ö.; Aksoy, U.; Kermeoglu, F.; Kalender, A.; Savtekin, G.; Ozkayalar, H.; Sayiner, S. Protective Effect of Alpha-lipoic Acid against Apical Periodontitis-induced Cardiac Injury in Rats. Eur. J. Oral Sci. 2019, 127, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wu, Q.; Tam, P.K.H. Immunomodulatory Mechanisms of Mesenchymal Stem Cells and Their Potential Clinical Applications. Int. J. Mol. Sci. 2022, 23, 10023. [Google Scholar] [CrossRef] [PubMed]
- Naji, A.; Favier, B.; Deschaseaux, F.; Rouas-Freiss, N.; Eitoku, M.; Suganuma, N. Mesenchymal Stem/Stromal Cell Function in Modulating Cell Death. Stem Cell Res. Ther. 2019, 10, 56. [Google Scholar] [CrossRef]
- Wu, X.; Jiang, J.; Gu, Z.; Zhang, J.; Chen, Y.; Liu, X. Mesenchymal Stromal Cell Therapies: Immunomodulatory Properties and Clinical Progress. Stem Cell Res. Ther. 2020, 11, 345. [Google Scholar] [CrossRef]
- Gallet, R.; Dawkins, J.; Valle, J.; Simsolo, E.; De Couto, G.; Middleton, R.; Tseliou, E.; Luthringer, D.; Kreke, M.; Smith, R.R. Exosomes Secreted by Cardiosphere-Derived Cells Reduce Scarring, Attenuate Adverse Remodelling, and Improve Function in Acute and Chronic Porcine Myocardial Infarction. Eur. Heart J. 2017, 38, 201–211. [Google Scholar] [CrossRef]
- Gullestad, L.; Ueland, T.; Vinge, L.E.; Finsen, A.; Yndestad, A.; Aukrust, P. Inflammatory Cytokines in Heart Failure: Mediators and Markers. Cardiology 2012, 122, 23–35. [Google Scholar] [CrossRef]
- Collins, K.A.; Korcarz, C.E.; Shroff, S.G.; Bednarz, J.E.; Fentzke, R.C.; Lin, H.; Leiden, J.M.; Lang, R.M. Accuracy of Echocardiographic Estimates of Left Ventricular Mass in Mice. Am. J. Physiol. Circ. Physiol. 2001, 280, H1954–H1962. [Google Scholar] [CrossRef]
- Scherrer-Crosbie, M.; Thibault, H.B. Echocardiography in Translational Research: Of Mice and Men. J. Am. Soc. Echocardiogr. 2008, 21, 1083–1092. [Google Scholar] [CrossRef]
- Vlahović, A.; Popović, A.D. Evaluation of Left Ventricular Diastolic Function Using Doppler Echocardiography. Med. Pregl. 1999, 52, 13–18. [Google Scholar]
- Ribeiro, A.B.; Santos-Junior, N.N.; Luiz, J.P.M.; de Oliveira, M.; Kanashiro, A.; Taira, T.M.; Fukada, S.Y.; Alves-Filho, J.C.; Fazan Junior, R.; Salgado, H.C. Cardiovascular and Autonomic Dysfunction in Murine Ligature-Induced Periodontitis. Sci. Rep. 2020, 10, 6891. [Google Scholar] [CrossRef] [PubMed]
- Elhaieg, A.; Farag, A.; Elfadadny, A.; Yokoi, A.; Hendawy, H.; Mandour, A.S.; Tanaka, R. Effect of Experimental Periodontitis on Cardiac Functions: A Comprehensive Study Using Echocardiography, Hemodynamic Analysis, and Histopathological Evaluation in a Rat Model. Front. Vet. Sci. 2023, 10, 1327484. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. Inflammation and Cardiovascular Disease Mechanisms. Am. J. Clin. Nutr. 2006, 83, 456S–460S. [Google Scholar] [CrossRef] [PubMed]
- Tonetti, M.S.; Van Dyke, T.E.; Working Group 1 of the Joint EFP/AAP Workshop. Periodontitis and Atherosclerotic Cardiovascular Disease: Consensus Report of the Joint EFP/AAPWorkshop on Periodontitis and Systemic Diseases. J. Periodontol. 2013, 84, S24–S29. [Google Scholar] [CrossRef]
- Angeli, F.; Verdecchia, P.; Pellegrino, C.; Pellegrino, R.G.; Pellegrino, G.; Prosciutti, L.; Giannoni, C.; Cianetti, S.; Bentivoglio, M. Association between Periodontal Disease and Left Ventricle Mass in Essential Hypertension. Hypertension 2003, 41, 488–492. [Google Scholar] [CrossRef]
- Shimazaki, Y.; Saito, T.; Kiyohara, Y.; Kato, I.; Kubo, M.; Iida, M.; Koga, T. Relationship between Electrocardiographic Abnormalities and Periodontal Disease: The Hisayama Study. J. Periodontol. 2004, 75, 791–797. [Google Scholar] [CrossRef]
- Schulze, A.; Kwast, S.; Pökel, C.; Busse, M. Assessment of the Relationship between Periodontitis and Cardiac Parameters in Patients with Early Chronic Heart Failure: A Cross-Sectional Study. J. Funct. Morphol. Kinesiol. 2024, 9, 52. [Google Scholar] [CrossRef]
- Gnecchi, M.; Zhang, Z.; Ni, A.; Dzau, V.J. Paracrine Mechanisms in Adult Stem Cell Signaling and Therapy. Circ. Res. 2008, 103, 1204–1219. [Google Scholar] [CrossRef]
- Golpanian, S.; Wolf, A.; Hatzistergos, K.E.; Hare, J.M. Rebuilding the Damaged Heart: Mesenchymal Stem Cells, Cell-Based Therapy, and Engineered Heart Tissue. Physiol. Rev. 2016, 96, 1127–1168. [Google Scholar] [CrossRef]
- Lee, R.H.; Pulin, A.A.; Seo, M.J.; Kota, D.J.; Ylostalo, J.; Larson, B.L.; Semprun-Prieto, L.; Delafontaine, P.; Prockop, D.J. Intravenous HMSCs Improve Myocardial Infarction in Mice Because Cells Embolized in Lung Are Activated to Secrete the Anti-Inflammatory Protein TSG-6. Cell Stem Cell 2009, 5, 54–63. [Google Scholar] [CrossRef]
- Liu, Z.; Mikrani, R.; Zubair, H.M.; Taleb, A.; Naveed, M.; Baig, M.M.F.A.; Zhang, Q.; Li, C.; Habib, M.; Cui, X. Systemic and Local Delivery of Mesenchymal Stem Cells for Heart Renovation: Challenges and Innovations. Eur. J. Pharmacol. 2020, 876, 173049. [Google Scholar] [CrossRef] [PubMed]
- Carr, C.A.; Stuckey, D.J.; Tatton, L.; Tyler, D.J.; Hale, S.J.M.; Sweeney, D.; Schneider, J.E.; Martin-Rendon, E.; Radda, G.K.; Harding, S.E. Bone Marrow-Derived Stromal Cells Home to and Remain in the Infarcted Rat Heart but Fail to Improve Function: An in Vivo Cine-MRI Study. Am. J. Physiol. Circ. Physiol. 2008, 295, H533–H542. [Google Scholar] [CrossRef] [PubMed]
- Barbash, I.M.; Chouraqui, P.; Baron, J.; Feinberg, M.S.; Etzion, S.; Tessone, A.; Miller, L.; Guetta, E.; Zipori, D.; Kedes, L.H. Systemic Delivery of Bone Marrow–Derived Mesenchymal Stem Cells to the Infarcted Myocardium: Feasibility, Cell Migration, and Body Distribution. Circulation 2003, 108, 863–868. [Google Scholar] [CrossRef] [PubMed]
- Zile, M.R.; Brutsaert, D.L. New Concepts in Diastolic Dysfunction and Diastolic Heart Failure: Part I: Diagnosis, Prognosis, and Measurements of Diastolic Function. Circulation 2002, 105, 1387–1393. [Google Scholar] [CrossRef]
- Öztürk, S.; Elçin, A.E.; Elçin, Y.M. Functions of Mesenchymal Stem Cells in Cardiac Repair. In Cell Biology and Translational Medicine, Volume 11: Stem Cell Therapy-Potential and Challenges; Springer: Berlin/Heidelberg, Germany, 2020; pp. 39–50. [Google Scholar]
- Singh, A.; Singh, A.; Sen, D. Mesenchymal Stem Cells in Cardiac Regeneration: A Detailed Progress Report of the Last 6 Years (2010–2015). Stem Cell Res. Ther. 2016, 7, 82. [Google Scholar] [CrossRef]
- Fan, M.; Huang, Y.; Chen, Z.; Xia, Y.; Chen, A.; Lu, D.; Wu, Y.; Zhang, N.; Qian, J. Efficacy of Mesenchymal Stem Cell Therapy in Systolic Heart Failure: A Systematic Review and Meta-Analysis. Stem Cell Res. Ther. 2019, 10, 150. [Google Scholar] [CrossRef]
- Nagaya, N.; Fujii, T.; Iwase, T.; Ohgushi, H.; Itoh, T.; Uematsu, M.; Yamagishi, M.; Mori, H.; Kangawa, K.; Kitamura, S. Intravenous Administration of Mesenchymal Stem Cells Improves Cardiac Function in Rats with Acute Myocardial Infarction through Angiogenesis and Myogenesis. Am. J. Physiol. Circ. Physiol. 2004, 287, H2670–H2676. [Google Scholar] [CrossRef]
- Eisner, D.A.; Caldwell, J.L.; Trafford, A.W.; Hutchings, D.C. The Control of Diastolic Calcium in the Heart: Basic Mechanisms and Functional Implications. Circ. Res. 2020, 126, 395–412. [Google Scholar] [CrossRef]
- Piątek-Matuszak, P.; Pasławski, R.; Pasławska, U.; Kiczak, L.; Płóciennik, M.; Janiszewski, A.; Michałek, M.; Gwizdała, A.; Kaźmierczak, J.; Gorący, J. Assessment of Myocardial Diastolic Dysfunction as a Result of Myocardial Infarction and Extracellular Matrix Regulation Disorders in the Context of Mesenchymal Stem Cell Therapy. J. Clin. Med. 2022, 11, 5430. [Google Scholar] [CrossRef]
- Fan, D.; Takawale, A.; Lee, J.; Kassiri, Z. Cardiac Fibroblasts, Fibrosis and Extracellular Matrix Remodeling in Heart Disease. Fibrogenesis Tissue Repair 2012, 5, 15. [Google Scholar] [CrossRef]
- Aboulgheit, A.; Karbasiafshar, C.; Sabra, M.; Zhang, Z.; Sodha, N.; Abid, M.R.; Sellke, F.W. Extracellular Vesicles Improve Diastolic Function and Substructure in Normal and High-Fat Diet Models of Chronic Myocardial Ischemia. J. Thorac. Cardiovasc. Surg. 2022, 164, e371–e384. [Google Scholar] [CrossRef] [PubMed]
- Tapp, H.; Deepe, R.; Ingram, J.A.; Kuremsky, M.; Hanley, E.N.; Gruber, H.E. Adipose-Derived Mesenchymal Stem Cells from the Sand Rat: Transforming Growth Factor Beta and 3D Co-Culture with Human Disc Cells Stimulate Proteoglycan and Collagen Type I Rich Extracellular Matrix. Arthritis Res. Ther. 2008, 10, R89. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Ye, Z.; Zhou, Y.; Tan, W.-S. Comparative Study of Mesenchymal Stem Cells from Rat Bone Marrow and Adipose Tissue. Turkish J. Biol. 2018, 42, 477–489. [Google Scholar] [CrossRef] [PubMed]
- Farag, A.; Mandour, A.S.; Hamabe, L.; Yoshida, T.; Shimada, K.; Tanaka, R. Novel Protocol to Establish the Myocardial Infarction Model in Rats Using a Combination of Medetomidine-Midazolam-Butorphanol (MMB) and Atipamezole. Front. Vet. Sci. 2022, 9, 1880. [Google Scholar] [CrossRef]
- Farag, A.; Mandour, A.S.; Hendawy, H.; Elhaieg, A.; Elfadadny, A.; Tanaka, R. A Review on Experimental Surgical Models and Anesthetic Protocols of Heart Failure in Rats. Front. Vet. Sci. 2023, 10, 386. [Google Scholar] [CrossRef]
- Kanazawa, M.; Atsuta, I.; Ayukawa, Y.; Yamaza, T.; Kondo, R.; Matsuura, Y.; Koyano, K. The Influence of Systemically or Locally Administered Mesenchymal Stem Cells on Tissue Repair in a Rat Oral Implantation Model. Int. J. Implant Dent. 2018, 4, 2. [Google Scholar] [CrossRef]
- Oliveira, M.O.A.; Leonço, Á.R.; Pavani, V.B.; Barbosa, I.R.; Campos, M.M. Omega-3 Effects on Ligature-Induced Periodontitis in Rats with Fructose-Induced Metabolic Syndrome. Inflammation 2023, 46, 388–403. [Google Scholar] [CrossRef]
- Holzhausen, M.; Rossa, C., Jr.; Marcantonio, E., Jr.; Nassar, P.O.; Spolidório, D.M.P.; Spolidório, L.C. Effect of Selective Cyclooxygenase-2 Inhibition on the Development of Ligature-induced Periodontitis in Rats. J. Periodontol. 2002, 73, 1030–1036. [Google Scholar] [CrossRef]
- Pontes Andersen, C.C.; Buschard, K.; Flyvbjerg, A.; Stoltze, K.; Holmstrup, P. Periodontitis Deteriorates Metabolic Control in Type 2 Diabetic Goto-Kakizaki Rats. J. Periodontol. 2006, 77, 350–356. [Google Scholar] [CrossRef]
- Shirakashi, D.J.; Leal, R.P.; Colombo, N.H.; Chiba, F.Y.; Garbin, C.A.S.; Jardim, E.G., Jr.; Antoniali, C.; Sumida, D.H. Maternal Periodontal Disease in Rats Decreases Insulin Sensitivity and Insulin Signaling in Adult Offspring. J. Periodontol. 2013, 84, 407–414. [Google Scholar] [CrossRef]
- Zacchigna, S.; Paldino, A.; Falcão-Pires, I.; Daskalopoulos, E.P.; Dal Ferro, M.; Vodret, S.; Lesizza, P.; Cannatà, A.; Miranda-Silva, D.; Lourenço, A.P. Towards Standardization of Echocardiography for the Evaluation of Left Ventricular Function in Adult Rodents: A Position Paper of the ESC Working Group on Myocardial Function. Cardiovasc. Res. 2021, 117, 43–59. [Google Scholar] [CrossRef]
- Lang, R.M.; Bierig, M.; Devereux, R.B.; Flachskampf, F.A.; Foster, E.; Pellikka, P.A.; Picard, M.H.; Roman, M.J.; Seward, J.; Shanewise, J.S. Recommendations for Chamber Quantification: A Report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, Developed in Conjunction with the European Association of Echocardiography, a Branch of the European Society of Cardiology. J. Am. Soc. Echocardiogr. 2005, 18, 1440–1463. [Google Scholar]



| Control | Model | Local | Systemic | |
|---|---|---|---|---|
| Bone Loss (mm) | 0.59 ± 0.14 | 1.46 ± 0.23 *** | 0.82 ± 0.069 ns | 1.11 ± 0.07 * |
| Bone Support (%) | 59.51 ± 5.37 | 30.33 ± 2.95 *** | 50.20 ± 3.02 ns | 45.87 ± 3.84 ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elhaieg, A.; Farag, A.; Koung Ngeun, S.; Kaneda, M.; Yokoi, A.; Mandour, A.S.; Tanaka, R. Therapeutic Potential of Local and Systemic Adipose-Derived Mesenchymal Stem Cell Injections in a Rat Model of Experimental Periodontitis: Implications for Cardiac Function. Int. J. Mol. Sci. 2025, 26, 3984. https://doi.org/10.3390/ijms26093984
Elhaieg A, Farag A, Koung Ngeun S, Kaneda M, Yokoi A, Mandour AS, Tanaka R. Therapeutic Potential of Local and Systemic Adipose-Derived Mesenchymal Stem Cell Injections in a Rat Model of Experimental Periodontitis: Implications for Cardiac Function. International Journal of Molecular Sciences. 2025; 26(9):3984. https://doi.org/10.3390/ijms26093984
Chicago/Turabian StyleElhaieg, Asmaa, Ahmed Farag, Sai Koung Ngeun, Masahiro Kaneda, Aimi Yokoi, Ahmed S. Mandour, and Ryou Tanaka. 2025. "Therapeutic Potential of Local and Systemic Adipose-Derived Mesenchymal Stem Cell Injections in a Rat Model of Experimental Periodontitis: Implications for Cardiac Function" International Journal of Molecular Sciences 26, no. 9: 3984. https://doi.org/10.3390/ijms26093984
APA StyleElhaieg, A., Farag, A., Koung Ngeun, S., Kaneda, M., Yokoi, A., Mandour, A. S., & Tanaka, R. (2025). Therapeutic Potential of Local and Systemic Adipose-Derived Mesenchymal Stem Cell Injections in a Rat Model of Experimental Periodontitis: Implications for Cardiac Function. International Journal of Molecular Sciences, 26(9), 3984. https://doi.org/10.3390/ijms26093984








