Piperazine-Substituted Pyranopyridines Exhibit Antiproliferative Activity and Act as Inhibitors of HBV Virion Production
Abstract
:1. Introduction
2. Results
2.1. Chemistry
2.2. Piperazine-Substituted Pyranopyridines Display Antiproliferative Activity
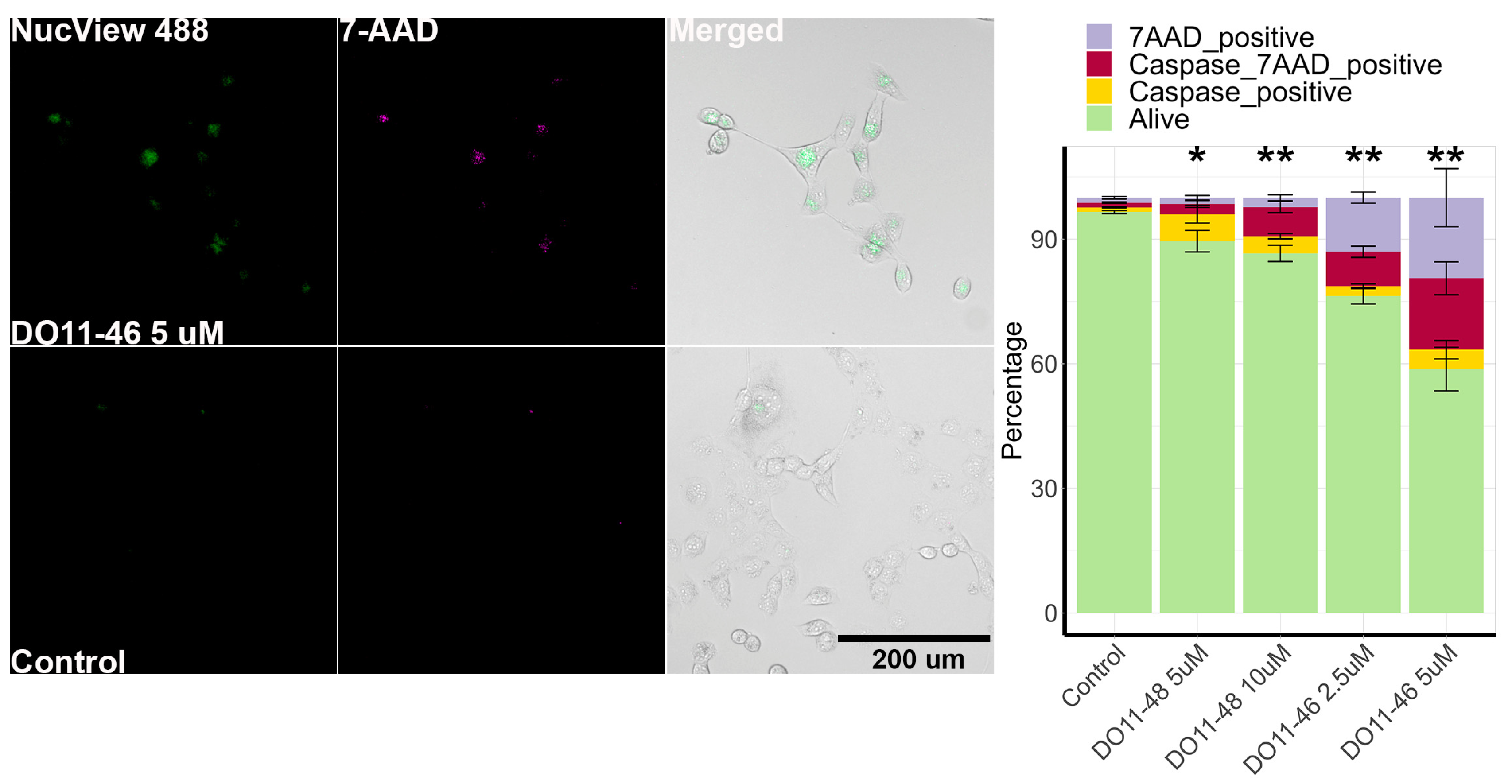
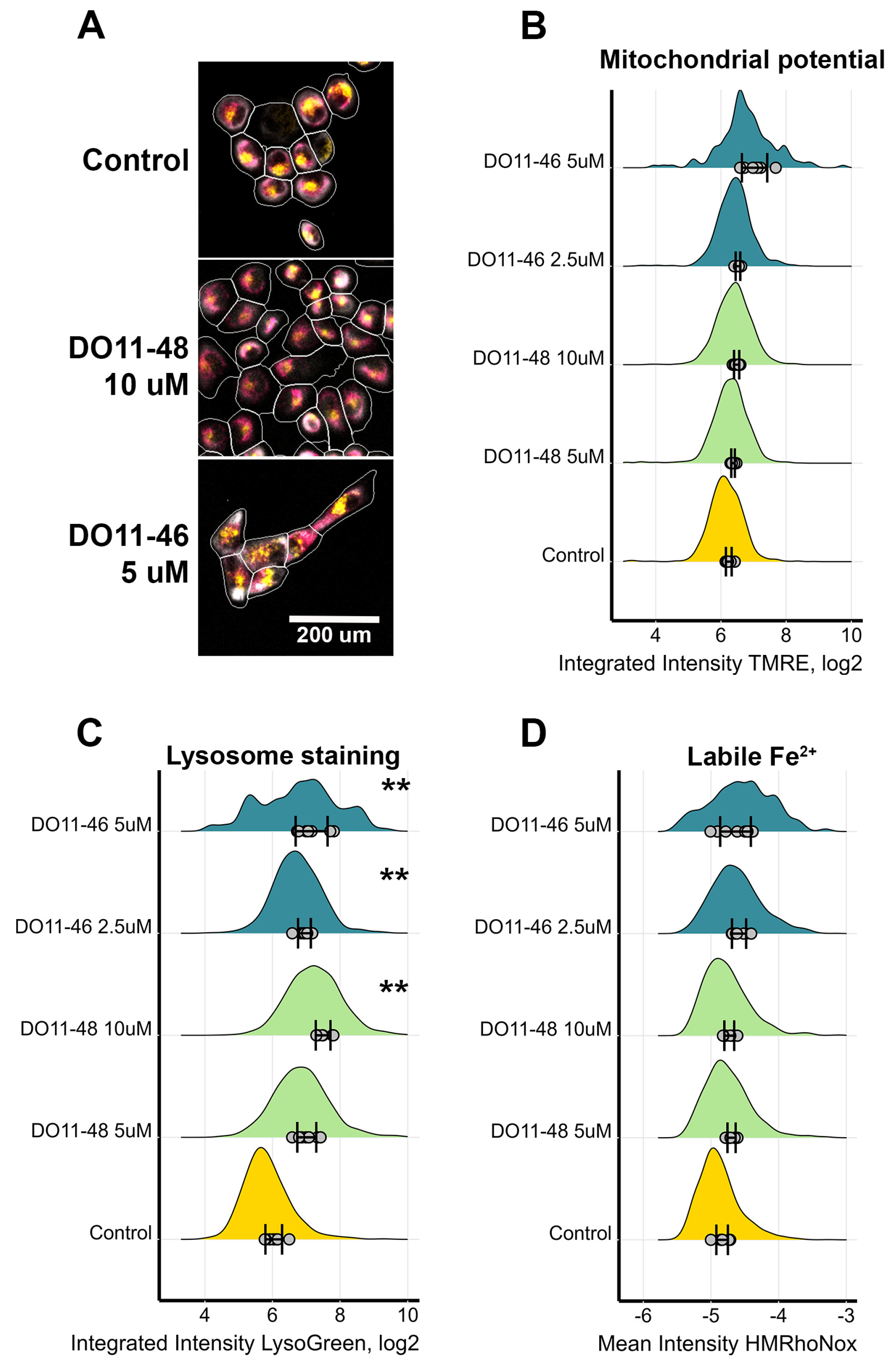
2.3. The Piperazine-Substituted Pyranopyridines Induce Apoptosis and Necrosis
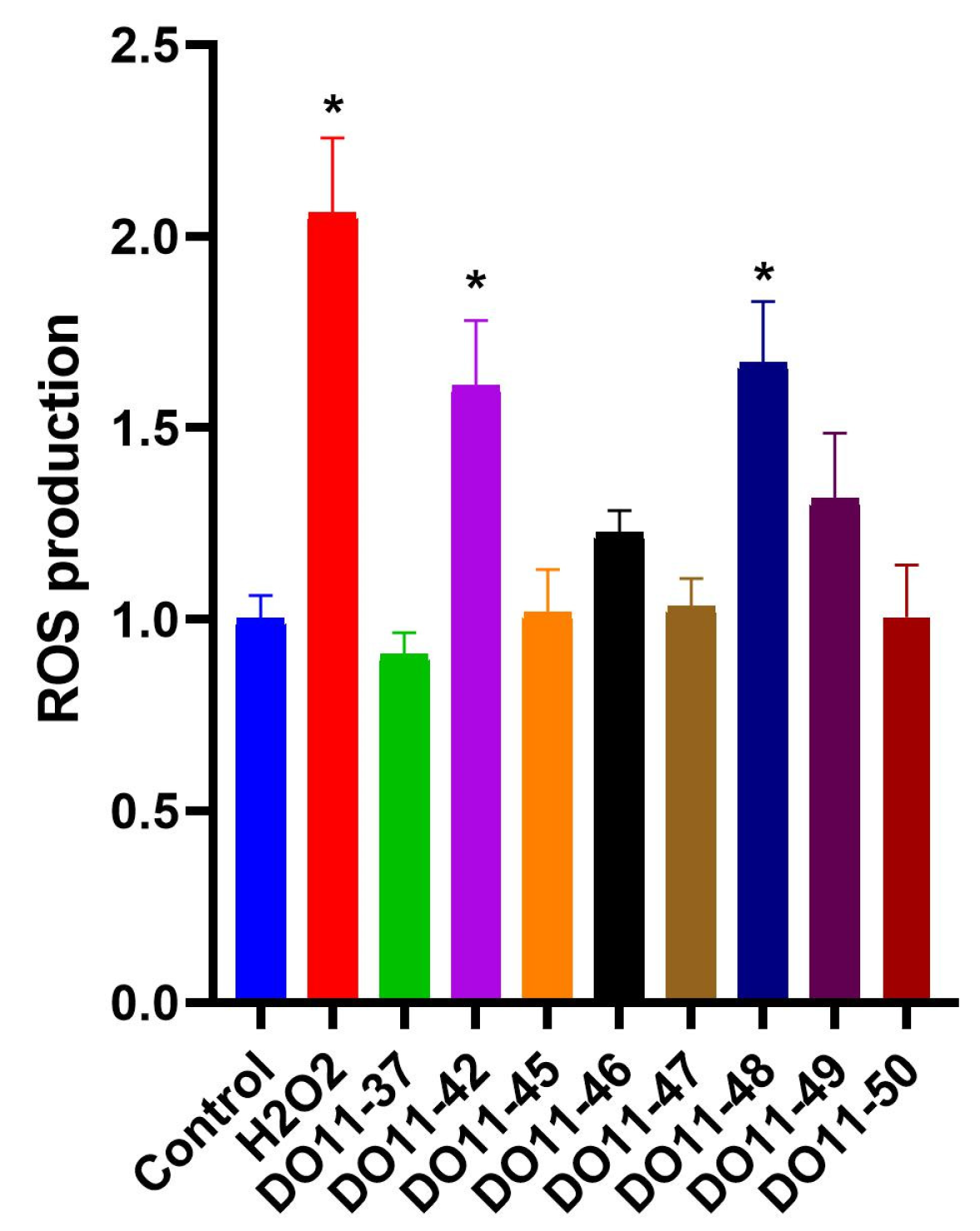
2.4. Intracellular ROS Assay
2.5. Antiviral Activity
3. Discussion
4. Materials and Methods
4.1. Reagents and Cell Lines
4.1.1. Chemistry
4.1.2. Biology
4.2. Synthesis of Compounds
4.3. Cell Cultivation and Antiproliferative Activity
4.4. Quantification of Production of Reactive Oxygen Species
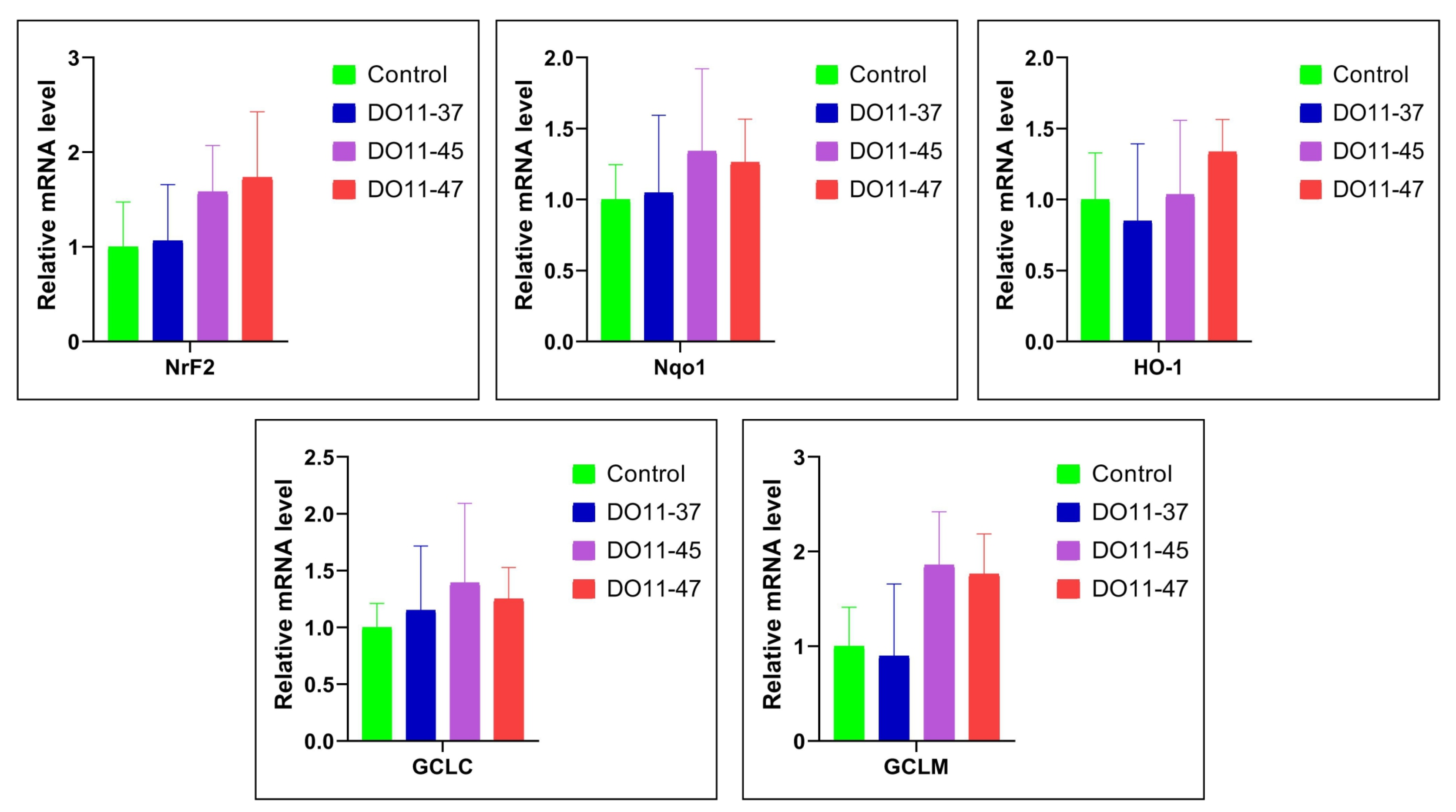
4.5. Status of Nrf2/ARE Pathway
4.6. Mitochondrial Membrane Potential and Lysosome Aggregation
4.7. Cell Death
4.8. Cell Cycle and ERK1/2 Activity Mesurements
4.9. Antiviral Activity
4.9.1. Influenza A Virus and SARS-CoV-2
4.9.2. Hepatitis B Virus (HBV)
4.9.3. Herpes Simplex Virus (HSV-1), Poliovirus (PV), and Newcastle Disease Virus (NDV)
4.9.4. Vaccinia Virus (VV)
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| COVID-19 | Coronavirus disease 19 |
| HIV | Human immunodeficiency virus |
| NCI | National cancer institute |
| IC50 | Inhibitory concentration |
| ERK | Extracellular signal-regulated protein kinase |
| SD | Standard deviation |
| ROS | Reactive oxygen species |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| Nqo1 | NAD(P)H:quinone dehydrogenase 1 |
| HO-1 | Heme oxygenase 1 |
| GCLC | Glutamate–cysteine ligase catalytic subunit |
| GCLM | Glutamate–cysteine ligase modifier subunit |
| ARE | Antioxidant response element |
| PV-1 | Poliovirus type 1 |
| NDV | Newcastle disease virus |
| HSV-1 | Herpes simplex virus type 1 |
| VV | Vaccinia virus |
| SAR | Structure–activity relationship |
| LMP | Lysosomal membrane permeability |
| RN | Regulated necrosis |
| PI3K | Phosphoinositide 3-kinase |
| mTOR | Mammalian target of rapamycin |
| TMS | Tetramethylsilane |
| ESI-MS | Electrospray ionization mass spectrometry |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Shah, R.; Battisti, N.M.L.; Brain, E.; Gnangnon, F.H.R.; Kanesvaran, R.; Mohile, S.; Noronha, V.; Puts, M.; Soto-Perez-de-Celis, E.; Pilleron, S. Updated cancer burden in oldest old: A population-based study using 2022 Globocan estimates. Cancer Epidemiol. 2024, 95, 102716. [Google Scholar] [CrossRef]
- Sapio, L.; Naviglio, S. Innovation through Tradition: The Current Challenges in Cancer Treatment. Int. J. Mol. Sci. 2022, 23, 5296. [Google Scholar] [CrossRef]
- Nurgali, K.; Rudd, J.A.; Was, H.; Abalo, R. Editorial: Cancer therapy: The challenge of handling a double-edged sword. Front. Pharmacol. 2022, 13, 1007762. [Google Scholar] [CrossRef] [PubMed]
- The global challenge of cancer. Nat. Cancer 2020, 1, 1–2. [CrossRef]
- Liu, B.; Zhou, H.; Tan, L.; Siu, K.T.H.; Guan, X.-Y. Exploring treatment options in cancer: Tumor treatment strategies. Signal Transduct. Target. Ther. 2024, 9, 175. [Google Scholar] [CrossRef] [PubMed]
- von Delft, A.; Hall, M.D.; Kwong, A.D.; Purcell, L.A.; Saikatendu, K.S.; Schmitz, U.; Tallarico, J.A.; Lee, A.A. Accelerating antiviral drug discovery: Lessons from COVID-19. Nat. Rev. Drug Discov. 2023, 22, 585–603. [Google Scholar] [CrossRef]
- Zhou, Z.; Liu, X.; Kang, D. Antiviral Drug Discovery. Int. J. Mol. Sci. 2024, 25, 7413. [Google Scholar] [CrossRef] [PubMed]
- Manns, M.P.; Maasoumy, B. Breakthroughs in hepatitis C research: From discovery to cure. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 533–550. [Google Scholar] [CrossRef]
- Montaner, J.S.; Lima, V.D.; Harrigan, P.R.; Lourenço, L.; Yip, B.; Nosyk, B.; Wood, E.; Kerr, T.; Shannon, K.; Moore, D.; et al. Expansion of HAART coverage is associated with sustained decreases in HIV/AIDS morbidity, mortality and HIV transmission: The “HIV Treatment as Prevention” experience in a Canadian setting. PLoS ONE 2014, 9, e87872. [Google Scholar] [CrossRef]
- Meunier, B. Hybrid molecules with a dual mode of action: Dream or reality? Acc. Chem. Res. 2008, 41, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Sirakanyan, S.N.; Spinelli, D.; Petrou, A.; Geronikaki, A.; Kartsev, V.G.; Hakobyan, E.K.; Yegoryan, H.A.; Zuppiroli, L.; Zuppiroli, R.; Ayvazyan, A.G.; et al. New Bicyclic Pyridine-Based Hybrids Linked to the 1,2,3-Triazole Unit: Synthesis via Click Reaction and Evaluation of Neurotropic Activity and Molecular Docking. Molecules 2023, 28, 921. [Google Scholar] [CrossRef] [PubMed]
- Tsogoeva, S.B. Recent progress in the development of synthetic hybrids of natural or unnatural bioactive compounds for medicinal chemistry. Mini Rev. Med. Chem. 2010, 10, 773–793. [Google Scholar] [CrossRef]
- Romanelli, M.N.; Manetti, D.; Braconi, L.; Dei, S.; Gabellini, A.; Teodori, E. The piperazine scaffold for novel drug discovery efforts: The evidence to date. Expert Opin. Drug Discov. 2022, 17, 969–984. [Google Scholar] [CrossRef]
- Chaudhary, P.; Kumar, R.; Verma, A.K.; Singh, D.; Yadav, V.; Chhillar, A.K.; Sharma, G.L.; Chandra, R. Synthesis and antimicrobial activity of N-alkyl and N-aryl piperazine derivatives. Bioorg. Med. Chem. 2006, 14, 1819–1826. [Google Scholar] [CrossRef]
- Thamban Chandrika, N.; Shrestha, S.K.; Ngo, H.X.; Tsodikov, O.V.; Howard, K.C.; Garneau-Tsodikova, S. Alkylated Piperazines and Piperazine-Azole Hybrids as Antifungal Agents. J. Med. Chem. 2018, 61, 158–173. [Google Scholar] [CrossRef]
- Kimura, M.; Masuda, T.; Yamada, K.; Kawakatsu, N.; Kubota, N.; Mitani, M.; Kishii, K.; Inazu, M.; Kiuchi, Y.; Oguchi, K.; et al. Antioxidative activities of novel diphenylalkyl piperazine derivatives with high affinities for the dopamine transporter. Bioorg. Med. Chem. Lett. 2004, 14, 4287–4290. [Google Scholar] [CrossRef]
- Jianzhi, S.; Qizeng, W.; Bin, L.; Wenhui, L.; Yunpeng, C.; Chenrong, F.; Lin, Z.; Huiting, C. Piperazine ferulate exerts antihypertensive effect and improves endothelial function in vitro and in vivo via the activation of endothelial nitric oxide synthase. Cell Mol. Biol. 2019, 65, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.R.; Sahu, B.; Pathania, S.; Singh, P.K.; Akhtar, M.J.; Kumar, B. Piperazine, a Key Substructure for Antidepressants: Its Role in Developments and Structure-Activity Relationships. ChemMedChem 2021, 16, 1878–1901. [Google Scholar] [CrossRef]
- Wang, T.; Kadow, J.F.; Zhang, Z.; Yin, Z.; Gao, Q.; Wu, D.; Parker, D.D.; Yang, Z.; Zadjura, L.; Robinson, B.A.; et al. Inhibitors of HIV-1 attachment. Part 4: A study of the effect of piperazine substitution patterns on antiviral potency in the context of indole-based derivatives. Bioorg. Med. Chem. Lett. 2009, 19, 5140–5145. [Google Scholar] [CrossRef]
- Marona, H.; Gunia, A.; Słoczyńska, K.; Rapacz, A.; Filipek, B.; Cegła, M.; Opoka, W. Preliminary evaluation of anticonvulsant activity and neurotoxicity of some 1,4-substituted piperazine derivatives. Acta Pol. Pharm. 2009, 66, 571–578. [Google Scholar] [PubMed]
- Piemontese, L.; Tomás, D.; Hiremathad, A.; Capriati, V.; Candeias, E.; Cardoso, S.M.; Chaves, S.; Santos, M.A. Donepezil structure-based hybrids as potential multifunctional anti-Alzheimer’s drug candidates. J. Enzyme Inhib. Med. Chem. 2018, 33, 1212–1224. [Google Scholar] [CrossRef] [PubMed]
- Ananda Kumar, C.S.; Benaka Prasad, S.B.; Vinaya, K.; Chandrappa, S.; Thimmegowda, N.R.; Kumar, Y.C.; Swarup, S.; Rangappa, K.S. Synthesis and in vitro antiproliferative activity of novel 1-benzhydrylpiperazine derivatives against human cancer cell lines. Eur. J. Med. Chem. 2009, 44, 1223–1229. [Google Scholar] [CrossRef]
- Sharma, V.; Das, R.; Sharma, D.; Mujwar, S.; Mehta, D.K. Green chemistry approach towards Piperazine: Anticancer agents. J. Mol. Struct. 2023, 1292, 136089. [Google Scholar] [CrossRef]
- Tahir, T.; Ashfaq, M.; Saleem, M.; Rafiq, M.; Shahzad, M.I.; Kotwica-Mojzych, K.; Mojzych, M. Pyridine Scaffolds, Phenols and Derivatives of Azo Moiety: Current Therapeutic Perspectives. Molecules 2021, 26, 4872. [Google Scholar] [CrossRef]
- Desai, N.C.; Kotadiya, G.M.; Trivedi, A.R. Studies on molecular properties prediction, antitubercular and antimicrobial activities of novel quinoline based pyrimidine motifs. Bioorg. Med. Chem. Lett. 2014, 24, 3126–3130. [Google Scholar] [CrossRef]
- Bernardino, A.M.R.; de Azevedo, A.R.; Pinheiro, L.C.d.S.; Borges, J.C.; Carvalho, V.L.; Miranda, M.D.; de Meneses, M.D.F.; Nascimento, M.; Ferreira, D.; Rebello, M.A.; et al. Synthesis and antiviral activity of new 4-(phenylamino)/4-[(methylpyridin-2-yl)amino]-1-phenyl-1H-pyrazolo[3,4-b]pyridine-4-carboxylic acids derivatives. Med. Chem. Res. 2007, 16, 352–369. [Google Scholar] [CrossRef]
- Chaban, T.; Ogurtsov, V.; Mahlovanyy, A.; Sukhodolska, N.; Chaban, I.; Harkov, S.; Matiychuk, V. Antioxidant properties of some novel derivatives thiazolo[4,5-b] pyridine. Pharmacia 2019, 66, 171–180. [Google Scholar] [CrossRef]
- Myriagkou, M.; Papakonstantinou, E.; Deligiannidou, G.E.; Patsilinakos, A.; Kontogiorgis, C.; Pontiki, E. Novel Pyrimidine Derivatives as Antioxidant and Anticancer Agents: Design, Synthesis and Molecular Modeling Studies. Molecules 2023, 28, 3913. [Google Scholar] [CrossRef]
- Xie, F.; Zhao, H.; Zhao, L.; Lou, L.; Hu, Y. Synthesis and biological evaluation of novel 2,4,5-substituted pyrimidine derivatives for anticancer activity. Bioorg. Med. Chem. Lett. 2009, 19, 275–278. [Google Scholar] [CrossRef]
- Bassyouni, F.; Tarek, M.; Salama, A.; Ibrahim, B.; Salah El Dine, S.; Yassin, N.; Hassanein, A.; Moharam, M.; Abdel-Rehim, M. Promising Antidiabetic and Antimicrobial Agents Based on Fused Pyrimidine Derivatives: Molecular Modeling and Biological Evaluation with Histopathological Effect. Molecules 2021, 26, 2370. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, S.R.; Ebrahimzadeh, M.A. Antiviral Activities of Pyridine Fused and Pyridine Containing Heterocycles, A Review (from 2000 to 2020). Mini Rev. Med. Chem. 2021, 21, 2584–2611. [Google Scholar] [CrossRef] [PubMed]
- Sangwan, S.; Yadav, N.; Kumar, R.; Chauhan, S.; Dhanda, V.; Walia, P.; Duhan, A. A score years’ update in the synthesis and biological evaluation of medicinally important 2-pyridones. Eur. J. Med. Chem. 2022, 232, 114199. [Google Scholar] [CrossRef] [PubMed]
- Romanelli, M.N.; Braconi, L.; Gabellini, A.; Manetti, D.; Marotta, G.; Teodori, E. Synthetic Approaches to Piperazine-Containing Drugs Approved by FDA in the Period of 2011–2023. Molecules 2024, 29, 68. [Google Scholar] [CrossRef]
- Sayed, M.T.M.; Hassan, R.A.; Halim, P.A.; El-Ansary, A.K. Recent updates on thienopyrimidine derivatives as anticancer agents. Med. Chem. Res. 2023, 32, 659–681. [Google Scholar] [CrossRef]
- Paronikyan, E.G.; Mirzoyan, G.V.; Noravyan, A.S.; Avakimyan, D.A.; Ter-Zakharyan, Y.Z. Synthesis and antibacterial activity of pyrano-(thiopyrano)[3,4-c]pyridine-, 2,7-naphthyridine-, and 5,6,7,8-tetrahydro-isoquinoline-3(2h)-thione derivatives. Pharm. Chem. J. 1993, 27, 759–762. [Google Scholar] [CrossRef]
- Cerec, V.; Glaise, D.; Garnier, D.; Morosan, S.; Turlin, B.; Drenou, B.; Gripon, P.; Kremsdorf, D.; Guguen-Guillouzo, C.; Corlu, A. Transdifferentiation of hepatocyte-like cells from the human hepatoma HepaRG cell line through bipotent progenitor. Hepatology 2007, 45, 957–967. [Google Scholar] [CrossRef]
- Ivanova, O.N.; Snezhkina, A.V.; Krasnov, G.S.; Valuev-Elliston, V.T.; Khomich, O.A.; Khomutov, A.R.; Keinanen, T.A.; Alhonen, L.; Bartosch, B.; Kudryavtseva, A.V.; et al. Activation of Polyamine Catabolism by N(1),N(11)-Diethylnorspermine in Hepatic HepaRG Cells Induces Dedifferentiation and Mesenchymal-Like Phenotype. Cells 2018, 7, 275. [Google Scholar] [CrossRef]
- Kanebratt, K.P.; Andersson, T.B. Evaluation of HepaRG cells as an in vitro model for human drug metabolism studies. Drug Metab. Dispos. 2008, 36, 1444–1452. [Google Scholar] [CrossRef]
- Viitanen, M.I.; Vasala, A.; Neubauer, P.; Alatossava, T. Cheese whey-induced high-cell-density production of recombinant proteins in Escherichia coli. Microb. Cell Fact. 2003, 2, 2. [Google Scholar] [CrossRef]
- Mikheeva, A.M.; Bogomolov, M.A.; Gasca, V.A.; Sementsov, M.V.; Spirin, P.V.; Prassolov, V.S.; Lebedev, T.D. Improving the power of drug toxicity measurements by quantitative nuclei imaging. Cell Death Discov. 2024, 10, 181. [Google Scholar] [CrossRef]
- Pachitariu, M.; Stringer, C. Cellpose 2.0: How to train your own model. Nat. Methods 2022, 19, 1634–1641. [Google Scholar] [CrossRef] [PubMed]
- Stirling, D.R.; Swain-Bowden, M.J.; Lucas, A.M.; Carpenter, A.E.; Cimini, B.A.; Goodman, A. CellProfiler 4: Improvements in speed, utility and usability. BMC Bioinform. 2021, 22, 433. [Google Scholar] [CrossRef] [PubMed]
- Grant, G.D.; Kedziora, K.M.; Limas, J.C.; Cook, J.G.; Purvis, J.E. Accurate delineation of cell cycle phase transitions in living cells with PIP-FUCCI. Cell Cycle 2018, 17, 2496–2516. [Google Scholar] [CrossRef]
- Regot, S.; Hughey, J.J.; Bajar, B.T.; Carrasco, S.; Covert, M.W. High-sensitivity measurements of multiple kinase activities in live single cells. Cell 2014, 157, 1724–1734. [Google Scholar] [CrossRef]
- Lebedev, T.D.; Khabusheva, E.R.; Mareeva, S.R.; Ivanenko, K.A.; Morozov, A.V.; Spirin, P.V.; Rubtsov, P.M.; Snezhkina, A.V.; Kudryavtseva, A.V.; Sorokin, M.I.; et al. Identification of cell type-specific correlations between ERK activity and cell viability upon treatment with ERK1/2 inhibitors. J. Biol. Chem. 2022, 298, 102226. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, A.V.; Valuev-Elliston, V.T.; Ivanova, O.N.; Kochetkov, S.N.; Starodubova, E.S.; Bartosch, B.; Isaguliants, M.G. Oxidative Stress during HIV Infection: Mechanisms and Consequences. Oxid. Med. Cell. Longev. 2016, 2016, 8910396. [Google Scholar] [CrossRef]
- Duarte-Olivenza, C.; Moran, G.; Hurle, J.M.; Lorda-Diez, C.I.; Montero, J.A. Lysosomes, caspase-mediated apoptosis, and cytoplasmic activation of P21, but not cell senescence, participate in a redundant fashion in embryonic morphogenetic cell death. Cell Death Dis. 2023, 14, 813. [Google Scholar] [CrossRef]
- Aleksunes, L.M.; Manautou, J.E. Emerging role of Nrf2 in protecting against hepatic and gastrointestinal disease. Toxicol. Pathol. 2007, 35, 459–473. [Google Scholar] [CrossRef]
- Kaur, J.; Gulati, M.; Jha, N.K.; Disouza, J.; Patravale, V.; Dua, K.; Singh, S.K. Recent advances in developing polymeric micelles for treating cancer: Breakthroughs and bottlenecks in their clinical translation. Drug Discov. Today 2022, 27, 1495–1512. [Google Scholar] [CrossRef]
- Ioele, G.; Chieffallo, M.; Occhiuzzi, M.A.; De Luca, M.; Garofalo, A.; Ragno, G.; Grande, F. Anticancer Drugs: Recent Strategies to Improve Stability Profile, Pharmacokinetic and Pharmacodynamic Properties. Molecules 2022, 27, 5436. [Google Scholar] [CrossRef]
- Will, M.; Qin, A.C.R.; Toy, W.; Yao, Z.; Rodrik-Outmezguine, V.; Schneider, C.; Huang, X.; Monian, P.; Jiang, X.; de Stanchina, E.; et al. Rapid Induction of Apoptosis by PI3K Inhibitors Is Dependent upon Their Transient Inhibition of RAS–ERK Signaling. Cancer Discov. 2014, 4, 334–347. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Das, R.; Mehta, D.K.; Sharma, D.; Sahu, R.K. Exploring Quinolone Scaffold: Unravelling the Chemistry of Anticancer Drug Design. Mini Rev. Med. Chem. 2022, 22, 69–88. [Google Scholar] [CrossRef]
- Taylor, R.D.; MacCoss, M.; Lawson, A.D.G. Rings in Drugs. J. Med. Chem. 2014, 57, 5845–5859. [Google Scholar] [CrossRef] [PubMed]
- Ismail, P.; Yousif, A.; Harki, E. Alterations of Some Heavy Metals and Trace Elements Levels in Breast Cancer. Med. Chem. 2017, 7, 758–760. [Google Scholar] [CrossRef]
- Zhang, R.H.; Guo, H.Y.; Deng, H.; Li, J.; Quan, Z.S. Piperazine skeleton in the structural modification of natural products: A review. J. Enzyme Inhib. Med. Chem. 2021, 36, 1165–1197. [Google Scholar] [CrossRef] [PubMed]
- Abu-Aisheh, M.N.; Mustafa, M.S.; El-Abadelah, M.M.; Naffa, R.G.; Ismail, S.I.; Zihlif, M.A.; Taha, M.O.; Mubarak, M.S. Synthesis and biological activity assays of some new N1-(flavon-7-yl)amidrazone derivatives and related congeners. Eur. J. Med. Chem. 2012, 54, 65–74. [Google Scholar] [CrossRef]
- Patel, R.V.; Mistry, B.M.; Syed, R.; Parekh, N.M.; Shin, H.S. Sulfonylpiperazines based on a flavone as antioxidant and cytotoxic agents. Arch. Pharm. 2019, 352, e1900051. [Google Scholar] [CrossRef]
- Mao, Z.; Zheng, X.; Qi, Y.; Zhang, M.; Huang, Y.; Wan, C.; Rao, G. Synthesis and biological evaluation of novel hybrid compounds between chalcone and piperazine as potential antitumor agents. RSC Adv. 2016, 6, 7723–7727. [Google Scholar] [CrossRef]
- Lin, Y.; Hu, C.; Zheng, X.; Wang, X.; Wan, C.; Mao, Z. Synthesis and Anti-tumor Activities of Novel 4’-(N-Substitued-1-piperazinyl) chalcone Derivatives. Chin. J. Org. Chem. 2017, 37, 237–241. [Google Scholar] [CrossRef]
- Chen, H.; Xu, F.; Liang, X.; Xu, B.-B.; Yang, Z.-L.; He, X.-L.; Huang, B.-Y.; Yuan, M. Design, synthesis and biological evaluation of novel arylpiperazine derivatives on human prostate cancer cell lines. Bioorganic Med. Chem. Lett. 2015, 25, 285–287. [Google Scholar] [CrossRef]
- Zsoldos, B.; Nagy, N.; Donkó-Tóth, V.; Keglevich, P.; Weber, M.; Dékány, M.; Nehr-Majoros, A.; Szőke, É.; Helyes, Z.; Hazai, L. Novel Piperazine Derivatives of Vindoline as Anticancer Agents. Int. J. Mol. Sci. 2024, 25, 7929. [Google Scholar] [CrossRef]
- Xu, A.P.; Xu, L.B.; Smith, E.R.; Fleishman, J.S.; Chen, Z.-S.; Xu, X.-X. Cell death in cancer chemotherapy using taxanes. Front. Pharmacol. 2024, 14, 1338633. [Google Scholar] [CrossRef]
- Ricci, M.S.; Zong, W.X. Chemotherapeutic approaches for targeting cell death pathways. Oncologist 2006, 11, 342–357. [Google Scholar] [CrossRef]
- Najafov, A.; Chen, H.; Yuan, J. Necroptosis and Cancer. Trends Cancer 2017, 3, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Mebratu, Y.; Tesfaigzi, Y. How ERK1/2 activation controls cell proliferation and cell death: Is subcellular localization the answer? Cell Cycle 2009, 8, 1168–1175. [Google Scholar] [CrossRef] [PubMed]
- Balmanno, K.; Cook, S.J. Tumour cell survival signalling by the ERK1/2 pathway. Cell Death Differ. 2009, 16, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative stress and antioxidant defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef]
- Snezhkina, A.V.; Kudryavtseva, A.V.; Kardymon, O.L.; Savvateeva, M.V.; Melnikova, N.V.; Krasnov, G.S.; Dmitriev, A.A. ROS Generation and Antioxidant Defense Systems in Normal and Malignant Cells. Oxid. Med. Cell. Longev. 2019, 2019, 6175804. [Google Scholar] [CrossRef]
- Andreyev, A.Y.; Kushnareva, Y.E.; Starkov, A.A. Mitochondrial metabolism of reactive oxygen species. Biochemistry 2005, 70, 200–214. [Google Scholar] [CrossRef]
- Ross, W.N. Understanding calcium waves and sparks in central neurons. Nat. Rev. Neurosci. 2012, 13, 157–168. [Google Scholar] [CrossRef]
- Trybus, W.; Trybus, E.; Król, T. Lysosomes as a Target of Anticancer Therapy. Int. J. Mol. Sci. 2023, 24, 2176. [Google Scholar] [CrossRef] [PubMed]
- Alu, A.; Han, X.; Ma, X.; Wu, M.; Wei, Y.; Wei, X. The role of lysosome in regulated necrosis. Acta Pharm. Sin. B 2020, 10, 1880–1903. [Google Scholar] [CrossRef] [PubMed]
- Berghe, T.V.; Linkermann, A.; Jouan-Lanhouet, S.; Walczak, H.; Vandenabeele, P. Regulated necrosis: The expanding network of non-apoptotic cell death pathways. Nat. Rev. Mol. Cell Biol. 2014, 15, 135–147. [Google Scholar] [CrossRef]
- He, H.; Shao, X.; Li, Y.; Gihu, R.; Xie, H.; Zhou, J.; Yan, H. Targeting Signaling Pathway Networks in Several Malignant Tumors: Progresses and Challenges. Front. Pharmacol. 2021, 12, 675675. [Google Scholar] [CrossRef]
- Sirico, M.; D’Angelo, A.; Gianni, C.; Casadei, C.; Merloni, F.; De Giorgi, U. Current State and Future Challenges for PI3K Inhibitors in Cancer Therapy. Cancers 2023, 15, 703. [Google Scholar] [CrossRef]
- Shaw, R.J.; Cantley, L.C. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature 2006, 441, 424–430. [Google Scholar] [CrossRef]
- Mishra, R.; Patel, H.; Alanazi, S.; Kilroy, M.K.; Garrett, J.T. PI3K Inhibitors in Cancer: Clinical Implications and Adverse Effects. Int. J. Mol. Sci. 2021, 22, 3464. [Google Scholar] [CrossRef]
- Engelman, J.A. Targeting PI3K signalling in cancer: Opportunities, challenges and limitations. Nat. Rev. Cancer 2009, 9, 550–562. [Google Scholar] [CrossRef]
- Hanker, A.B.; Kaklamani, V.; Arteaga, C.L. Challenges for the Clinical Development of PI3K Inhibitors: Strategies to Improve Their Impact in Solid Tumors. Cancer Discov. 2019, 9, 482–491. [Google Scholar] [CrossRef]
- Crawford, A.; Fassett, R.G.; Geraghty, D.P.; Kunde, D.A.; Ball, M.J.; Robertson, I.K.; Coombes, J.S. Relationships between single nucleotide polymorphisms of antioxidant enzymes and disease. Gene 2012, 501, 89–103. [Google Scholar] [CrossRef]
- Li, H.; He, H.; Wang, Z.; Cai, J.; Sun, B.; Wu, Q.; Zhang, Y.; Zhou, G.; Yang, L. Rice protein suppresses ROS generation and stimulates antioxidant gene expression via Nrf2 activation in adult rats. Gene 2016, 585, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Wufuer, R.; Fan, Z.; Liu, K.; Zhang, Y. Differential Yet Integral Contributions of Nrf1 and Nrf2 in the Human HepG2 Cells on Antioxidant Cytoprotective Response against Tert-Butylhydroquinone as a Pro-Oxidative Stressor. Antioxidants 2021, 10, 1610. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Yang, F.; Teng, L.; Katayama, I. 6-Shogaol Protects Human Melanocytes against Oxidative Stress through Activation of the Nrf2-Antioxidant Response Element Signaling Pathway. Int. J. Mol. Sci. 2020, 21, 3537. [Google Scholar] [CrossRef]
- Nguyen, T.; Nioi, P.; Pickett, C.B. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J. Biol. Chem. 2009, 284, 13291–13295. [Google Scholar] [CrossRef] [PubMed]
- Correa-Padilla, E.; Hernández-Cano, A.; Cuevas, G.; Acevedo-Betancur, Y.; Esquivel-Guadarrama, F.; Martinez-Mayorga, K. Modifications in the piperazine ring of nucleozin affect anti-influenza activity. PLoS ONE 2023, 18, e0277073. [Google Scholar] [CrossRef]
- Enkhtaivan, G.; Kim, D.H.; Park, G.S.; Pandurangan, M.; Nicholas, D.A.; Moon, S.H.; Kadam, A.A.; Patel, R.V.; Shin, H.-S.; Mistry, B.M. Berberine-piperazine conjugates as potent influenza neuraminidase blocker. Int. J. Biol. Macromol. 2018, 119, 1204–1210. [Google Scholar] [CrossRef]
- Chu, Y.; Raja Sekhara Reddy, B.; Pratap Reddy Gajulapalli, V.; Sudhakar Babu, K.; Kim, E.; Lee, S. Design, synthesis, and biological evaluation of N-arylpiperazine derivatives as interferon inducers. Bioorg. Med. Chem. Lett. 2020, 30, 127613. [Google Scholar] [CrossRef]
- WHO Factsheet. Hepatitis B. Available online: https://www.who.int/news-room/fact-sheets/detail/hepatitis-b (accessed on 28 January 2025).
- Kim, K.-H.; Kim, N.D.; Seong, B.-L. Discovery and Development of Anti-HBV Agents and Their Resistance. Molecules 2010, 15, 5878–5908. [Google Scholar] [CrossRef]
- Yasutake, Y.; Hattori, S.-I.; Kumamoto, H.; Tamura, N.; Maeda, K.; Mitsuya, H. Deviated binding of anti-HBV nucleoside analog E-CFCP-TP to the reverse transcriptase active site attenuates the effect of drug-resistant mutations. Sci. Rep. 2024, 14, 15742. [Google Scholar] [CrossRef]
- Le, T.H.; Lipatova, A.V.; Volskaya, M.A.; Tikhonova, O.A.; Chumakov, P.M. The State of The Jak/Stat Pathway Affects the Sensitivity of Tumor Cells to Oncolytic Enteroviruses. Mol. Biol. 2020, 54, 570–577. [Google Scholar] [CrossRef]
- Lipatova, A.V.; Soboleva, A.V.; Gorshkov, V.A.; Bubis, J.A.; Solovyeva, E.M.; Krasnov, G.S.; Kochetkov, D.V.; Vorobyev, P.O.; Ilina, I.Y.; Moshkovskii, S.A.; et al. Multi-Omics Analysis of Glioblastoma Cells’ Sensitivity to Oncolytic Viruses. Cancers 2021, 13, 5268. [Google Scholar] [CrossRef]
- Vorobyev, P.O.; Kochetkov, D.V.; Chumakov, P.M.; Zakirova, N.F.; Zotova-Nefedorova, S.I.; Vasilenko, K.V.; Alekseeva, O.N.; Kochetkov, S.N.; Bartosch, B.; Lipatova, A.V.; et al. 2-Deoxyglucose, an Inhibitor of Glycolysis, Enhances the Oncolytic Effect of Coxsackievirus. Cancers 2022, 14, 5611. [Google Scholar] [CrossRef] [PubMed]
- Riss, T.D.L.; Moravec, R.A.; Niles, A.V.L.; Duellman, S.; Benink, H.A.; Worzella, T.J.; Minor, L. Cell Viability Assays. In Assay Guidance Manual; Markossian, S., Grossman, A., Arkin, M., Auld, D., Austin, C., Baell, J., Brimacombe, K., Chung, T.D.Y., Coussens, N.P., Dahlin, J.L., et al., Eds.; Eli Lilly & Company and the National Center for Advancing Translational Sciences: Bethesda, MD, USA, 2004. [Google Scholar]
- van Meerloo, J.; Kaspers, G.J.; Cloos, J. Cell sensitivity assays: The MTT assay. Methods Mol. Biol. 2011, 731, 237–245. [Google Scholar] [CrossRef]
- Aranda, A.; Sequedo, L.; Tolosa, L.; Quintas, G.; Burello, E.; Castell, J.V.; Gombau, L. Dichloro-dihydro-fluorescein diacetate (DCFH-DA) assay: A quantitative method for oxidative stress assessment of nanoparticle-treated cells. Toxicol. In Vitro 2013, 27, 954–963. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lin, E.; Johansen, M.J.; Madden, T.; Felix, E.; Martirosyan, K.S.; Frank, S.J. Reactive Oxygen Species Generation in Human Cells by a Novel Magnetic Resonance Imaging Contrast Agent. J. Toxicol. 2018, 2018, 6362426. [Google Scholar] [CrossRef] [PubMed]
- Golikov, M.V.; Karpenko, I.L.; Lipatova, A.V.; Ivanova, O.N.; Fedyakina, I.T.; Larichev, V.F.; Zakirova, N.F.; Leonova, O.G.; Popenko, V.I.; Bartosch, B.; et al. Cultivation of Cells in a Physiological Plasmax Medium Increases Mitochondrial Respiratory Capacity and Reduces Replication Levels of RNA Viruses. Antioxidants 2022, 11, 97. [Google Scholar] [CrossRef]
- Ivanova, O.N.; Gavlina, A.V.; Karpenko, I.L.; Zenov, M.A.; Antseva, S.S.; Zakirova, N.F.; Valuev-Elliston, V.T.; Krasnov, G.S.; Fedyakina, I.T.; Vorobyev, P.O.; et al. Polyamine Catabolism Revisited: Acetylpolyamine Oxidase Plays a Minor Role due to Low Expression. Cells 2024, 13, 1134. [Google Scholar] [CrossRef]
- Smith, S.M.; Wunder, M.A.B.; Norris, D.A.; Shellman, Y.G. A simple protocol for using a LDH-based cytotoxicity assay to assess the effects of death and growth inhibition at the same time. PLoS ONE 2011, 6, e26908. [Google Scholar] [CrossRef]
- Smirnova, O.A.; Ivanova, O.N.; Mukhtarov, F.; Valuev-Elliston, V.T.; Fedulov, A.P.; Rubtsov, P.M.; Zakirova, N.F.; Kochetkov, S.N.; Bartosch, B.; Ivanov, A.V. Hepatitis Delta Virus Antigens Trigger Oxidative Stress, Activate Antioxidant Nrf2/ARE Pathway, and Induce Unfolded Protein Response. Antioxidants 2023, 12, 974. [Google Scholar] [CrossRef]
- Alfaiate, D.; Lucifora, J.; Abeywickrama-Samarakoon, N.; Michelet, M.; Testoni, B.; Cortay, J.C.; Sureau, C.; Zoulim, F.; Dény, P.; Durantel, D. HDV RNA replication is associated with HBV repression and interferon-stimulated genes induction in super-infected hepatocytes. Antiviral Res. 2016, 136, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Shakiba, Y.; Vorobyev, P.O.; Naumenko, V.A.; Kochetkov, D.V.; Zajtseva, K.V.; Valikhov, M.P.; Yusubalieva, G.M.; Gumennaya, Y.D.; Emelyanov, E.A.; Semkina, A.S.; et al. Oncolytic Efficacy of a Recombinant Vaccinia Virus Strain Expressing Bacterial Flagellin in Solid Tumor Models. Viruses 2023, 15, 828. [Google Scholar] [CrossRef] [PubMed]
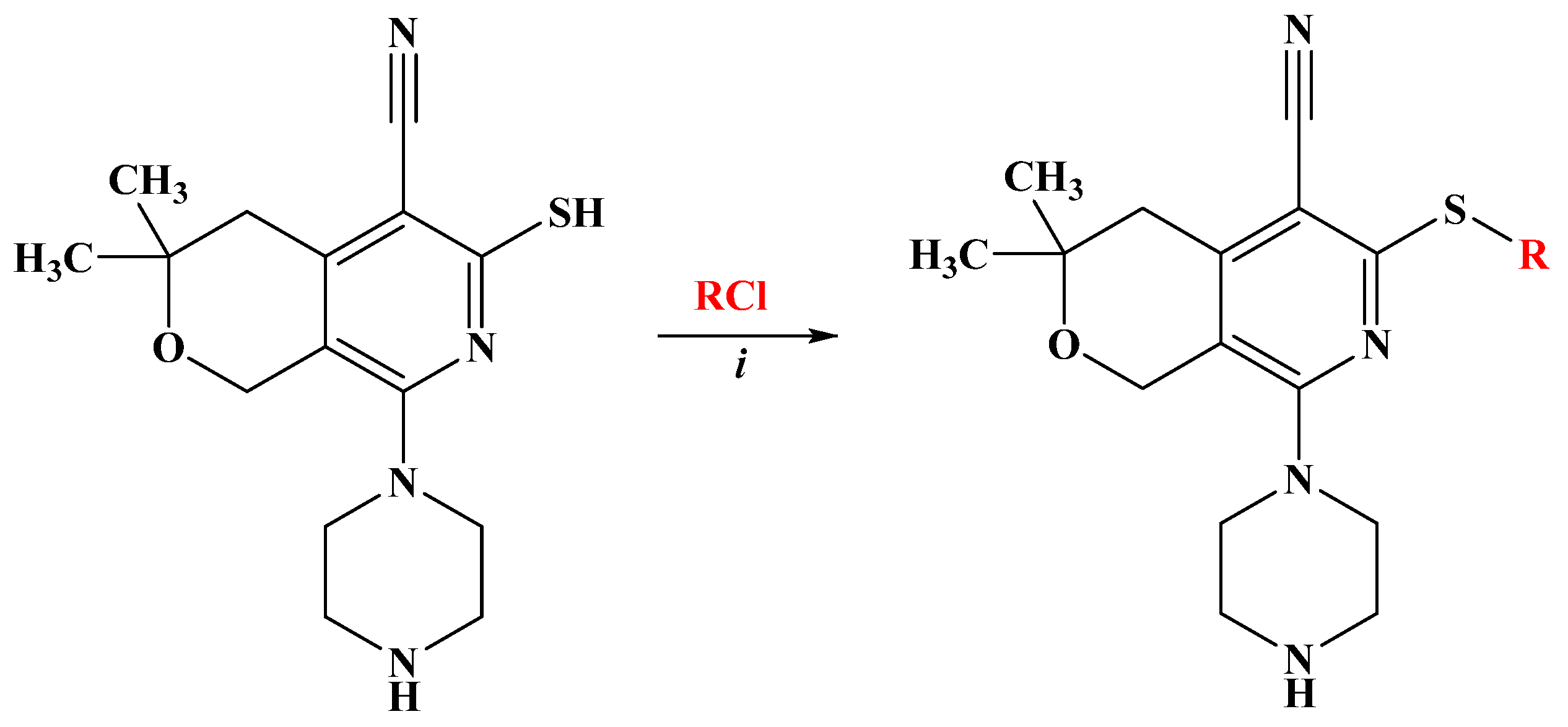
| Compounds N | R | Yield (%) * |
|---|---|---|
| DO11-37 | CH2CONH2 | 83.6 |
| DO11-42 | CH2-2-ClC6H4 | 97.2 |
| DO11-45 | CH2CONH-4-MeC6H4 | 97.9 |
| DO11-46 | CH2CONH-2,5-Cl2C6H3 | 73.8 |
| DO11-47 | CH2CONH-3-OMeC6H4 | 95.1 |
| DO11-48 | CH2CONH-2-OMeC6H4 | 95.8 |
| DO11-49 | CH2CONH-2,4-(OMe)2C6H3 | 78.8 |
| DO11-50 | CH2CONH-4-COMeC6H4 | 83.5 |
| Compound | IC50 (µM) | |||||||
|---|---|---|---|---|---|---|---|---|
| A549 | HeLa | DU145 | K562 | SH-SY5Y | GBM5522 | GBM6138 | HepaRG | |
| DO11-37 | 294.2 ± 35.9 | 133.2 ± 38.0 | 225.3 ± 52.4 | 91.4 ± 29.6 | 99.4 ± 21.4 | 309.4 ± 63.3 | 571.4 ± 77.7 | 967.9 ±87.8 |
| DO11-42 | 7.0 ± 1.9 | 1.9 ± 0.3 | 1.3 ± 0.2 | 1.5 ± 0.8 | 4.5 ± 1.8 | 4.2 ± 1.3 | 51.2 ± 10.7 | 24.5 ±2.8 |
| DO11-45 | 22.4 ± 6.8 | 21.9 ± 12.7 | 27.7 ± 0.6 | 5.6 ± 4.4 | 43.1 ± 11.0 | 13.5 ± 4.7 | 129.1 ± 52.8 | 73.7 ±15.5 |
| DO11-46 | 6.4 ± 0.8 | 1.9 ± 0.2 | 0.5 ± 0.6 | 1.7 ± 0.7 | 19.8 ± 2.2 | 3.2 ± 1.5 | 25.0 ± 12.0 | 15.2 ± 3.9 |
| DO11-47 | 67.8 ± 5.8 | 39.3 ± 16.9 | 10.0 ± 2.9 | 12.8 ± 4.2 | 36.7 ± 18.2 | 20.0 ± 6.8 | 103.8 ± 22.1 | 437.2 ± 14.8 |
| DO11-48 | 3.5 ± 0.7 | 2.2 ± 0.6 | 2.5 ± 1.2 | 0.5 ± 0.1 | 18.1 ± 5.1 | 3.8 ± 1.5 | 27.9 ± 2.6 | 10.9 ± 1.6 |
| DO11-49 | 2.0 ± 0.5 | 1.7 ± 0.4 | 1.1 ± 0.3 | 13.8 ± 3.8 | 13.3 ± 3.7 | 4.1 ± 0.4 | 3.8 ± 1.2 | 10.6 ± 1.2 |
| DO11-50 | 45.8 ± 12.6 | 74.5 ± 3.5 | 26.7 ± 8.4 | 45.6 ± 20.0 | 13.2 ± 4.3 | 15.9 ± 4.2 | 54.7 ± 28.7 | 42.4 ± 8.3 |
| 5-Fluorouracil | 1.2 ±0.3 | 1.3 ± 0.3 | 1.5 ± 0.2 | |||||
| Sorafenib | 40 ± 10 | 84 ± 14 | >100 | |||||
| Compound | Influenza A Virus | SARS-CoV-2 | PV1 | NDV | HSV-1 | VV | |||
|---|---|---|---|---|---|---|---|---|---|
| Δlgmax | IC50, µM | CC50, µM | Δlgmax | IC50, µM | IC50, µM | IC50, µM | IC50, µM | IC50, µM | |
| DO11-37 | 0.5 | >0.3 | 1.68 | 0.5 | >200 | >300 | >300 | >300 | >300 |
| DO11-42 | 0.5 | >0.019 | 0.44 | 0 | >15 | >7.5 | >7.5 | >7.5 | >7.5 |
| DO11-45 | 0 | >0.038 | 0.82 | 0 | >30 | >30 | >30 | >30 | >30 |
| DO11-46 | 0.5 | >0.019 | 0.40 | 1.0 | >8 | >6 | >6 | >6 | >6 |
| DO11-47 | 0 | >0.038 | 0.81 | 0 | > 38 | >60 | >60 | >60 | >60 |
| DO11-48 | 0 | >0.019 | 0.83 | 0 | >10 | >3.75 | >3.75 | >3.75 | >3.75 |
| DO11-49 | 0 | >0.3 | 0.90 | 0 | >10 | >3 | >3 | >3 | >3 |
| DO11-50 | 0 | >0.3 | 1.27 | 0 | >15 | >5 | >5 | >5 | >5 |
| N-HydroxyC | 5.0 | 0.5 | |||||||
| Oseltamivir | 6.0 | 0.02 | >10 | ||||||
| Compound | Extracellular HBV DNA (Δlog10) | Intracellular Total HBV RNA (Δlog10) | ||
|---|---|---|---|---|
| 1 µM | 10 µM | 1 µM | 10 µM | |
| DO11-37 | 1.97 ± 0.03 | 2.32 ± 0.16 | <0.5 | <0.5 |
| DO11-42 | 2.52 ± 0.36 | 2.31 ± 0.29 | <0.5 | 0.53 ± 0.31 |
| DO11-45 | 2.92 ± 0.44 | 3.31 ± 0.25 | <0.5 | <0.5 |
| DO11-46 | 2.61 ± 0.43 | 3.27 ± 0.14 | <0.5 | <0.5 |
| DO11-47 | <0.5 | <0.5 | <0.5 | <0.5 |
| DO11-48 | <0.5 | <0.5 | <0.5 | <0.5 |
| DO11-49 | <0.5 | <0.5 | <0.5 | <0.5 |
| DO11-50 | <0.5 | <0.5 | <0.5 | <0.5 |
| 3TC | 0.93 | 1.04 | <0.5 | <0.5 |
| Gene ID | Orientation | Sequence |
|---|---|---|
| Nrf2 | Forward | 5′-TACTCCCAGGTTGCCCACA-3′ |
| Reverse | 5′-CATCTACAAACGGGAATGTCTGC-3′ | |
| Nqo1 | Forward | 5′-CCGTGGATCCCTTGCAGAGA-3′ |
| Reverse | 5′-AGGACCCTTCCGGAGTAAGA-3′ | |
| HO-1 | Forward | 5′-CCAGCAACAAAGTGCAAGATTC-3′ |
| Reverse | 5′-TCACATGGCATAAAGCCCTACAG-3′ | |
| GCLC | Forward | 5′-GGATTTGGAAATGGGCAATTG-3′ |
| Reverse | 5′-CTCAGATATACTGCAGGCTTGGAA-3′ | |
| GCLM | Forward | 5′-TGCAGTTGACATGGCCTGTT-3′ |
| Reverse | 5′-TCACAGAATCCAGCTGTGCAA-3′ | |
| HBV tRNA | Forward | 5′-GCTGACGCAACCCCCACT-3′ |
| Reverse | 5′-AGGAGTTCCGCAGTATGG-3′ | |
| HBV DNA | Forward | 5′-AAATTCGCAGTCCCCAACCT-3′ |
| Reverse | 5′-CGCAGACACATCCAGCGATA-3′ | |
| GUS | Forward | 5′-CGTGGTTGGAGAGCTCATTTGGAA-3′ |
| Reverse | 5′-ATTCCCCAGCACTCTCGTCGGT-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buloyan, S.; Harutyunyan, A.; Gasparyan, H.; Sakeyan, A.; Shahkhatuni, A.; Zakirova, N.F.; Yusubalieva, G.; Kirillov, I.M.; Fedyakina, I.T.; Solyev, P.N.; et al. Piperazine-Substituted Pyranopyridines Exhibit Antiproliferative Activity and Act as Inhibitors of HBV Virion Production. Int. J. Mol. Sci. 2025, 26, 3991. https://doi.org/10.3390/ijms26093991
Buloyan S, Harutyunyan A, Gasparyan H, Sakeyan A, Shahkhatuni A, Zakirova NF, Yusubalieva G, Kirillov IM, Fedyakina IT, Solyev PN, et al. Piperazine-Substituted Pyranopyridines Exhibit Antiproliferative Activity and Act as Inhibitors of HBV Virion Production. International Journal of Molecular Sciences. 2025; 26(9):3991. https://doi.org/10.3390/ijms26093991
Chicago/Turabian StyleBuloyan, Sona, Arpine Harutyunyan, Hrachik Gasparyan, Anahit Sakeyan, Astghik Shahkhatuni, Natalia F. Zakirova, Gaukhar Yusubalieva, Ilya M. Kirillov, Irina T. Fedyakina, Pavel N. Solyev, and et al. 2025. "Piperazine-Substituted Pyranopyridines Exhibit Antiproliferative Activity and Act as Inhibitors of HBV Virion Production" International Journal of Molecular Sciences 26, no. 9: 3991. https://doi.org/10.3390/ijms26093991
APA StyleBuloyan, S., Harutyunyan, A., Gasparyan, H., Sakeyan, A., Shahkhatuni, A., Zakirova, N. F., Yusubalieva, G., Kirillov, I. M., Fedyakina, I. T., Solyev, P. N., Lipatova, A. V., Bogomolov, M. A., Prassolov, V. S., Lebedev, T. D., & Ivanov, A. V. (2025). Piperazine-Substituted Pyranopyridines Exhibit Antiproliferative Activity and Act as Inhibitors of HBV Virion Production. International Journal of Molecular Sciences, 26(9), 3991. https://doi.org/10.3390/ijms26093991









