Influence of Fatty Acid Desaturase Enzyme-1 Gene (FADS-1) Polymorphism on Serum Polyunsaturated Fatty Acids Levels, Desaturase Enzymes, Lipid Profile, and Glycemic Control Parameters in Newly Diagnosed Diabetic Mellitus Patients
Abstract
1. Introduction
2. Results
2.1. Assessment of Various Variables and Biochemical Markers in the Study Groups
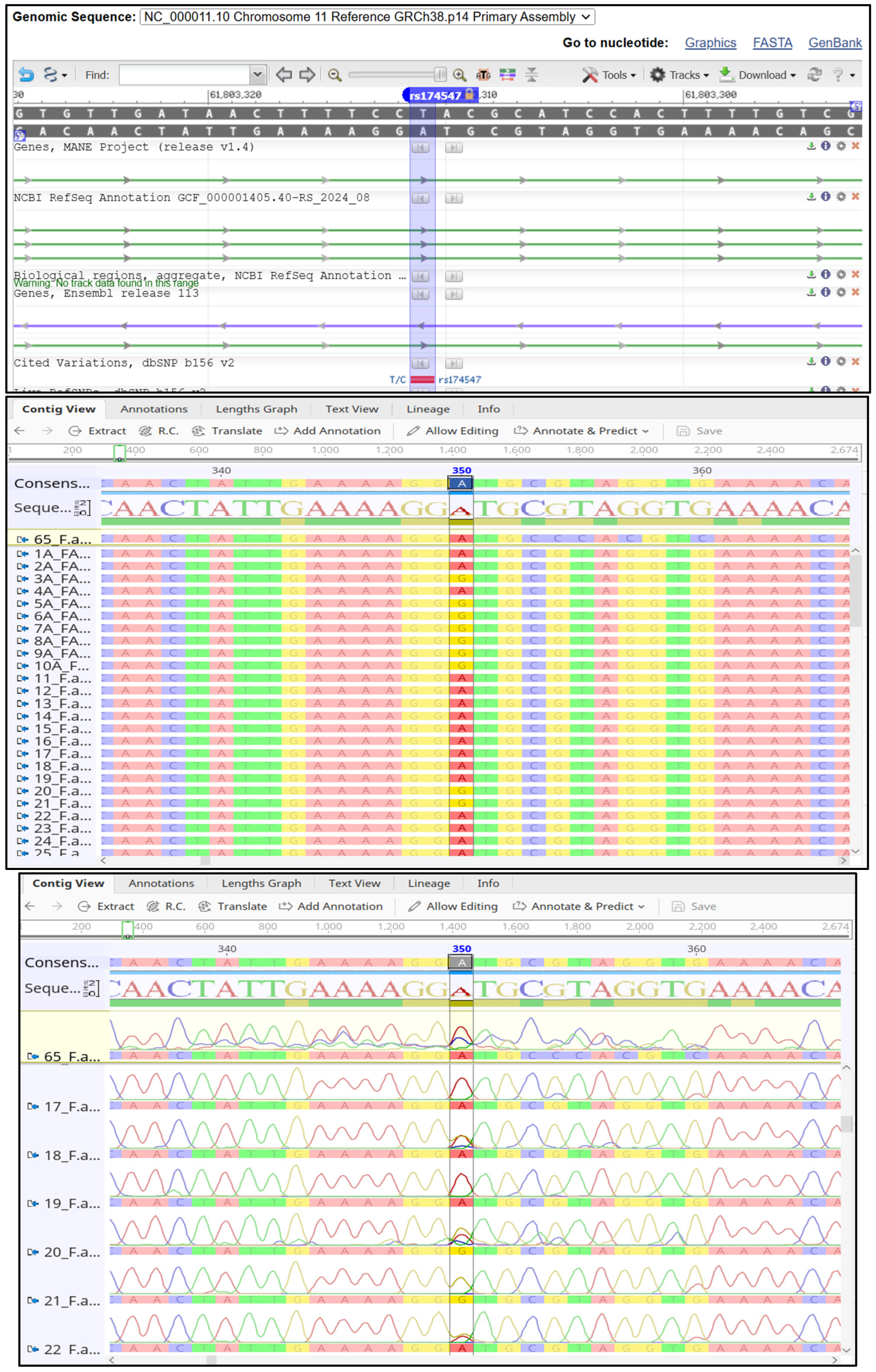
2.2. Genetic Analysis
2.3. Multivariate Analysis
2.4. Assessment of Lipid Profile, Apolipoproteins, and Inflammatory Mediator in Type 2 Diabetes Patients Under the Effect of rs174547 Polymorphism
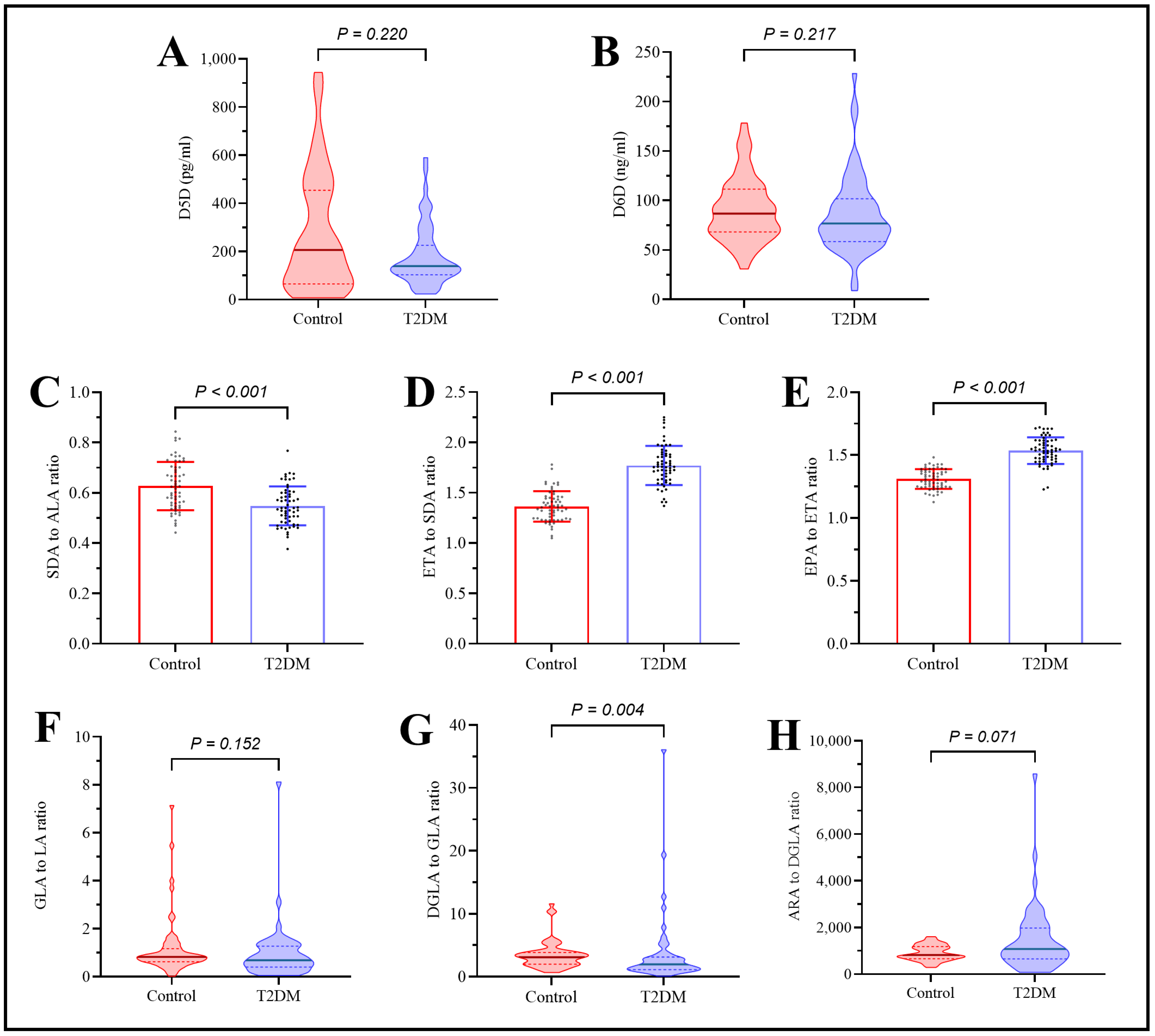
2.5. Assessment of Plasma Polyunsaturated Fatty Acids in Type 2 Diabetes Patients Under the Effect of rs174547 Polymorphism
2.6. Assessment of Desaturase Enzymes in Type 2 Diabetes Patients Under the Effect of rs174547 Polymorphism
3. Discussion
4. Methods
4.1. Study Design and Settings
4.2. Ethical Approval
4.3. Patient Selection and Data Collection
4.3.1. Inclusion Criteria
4.3.2. Exclusion Criteria
4.4. Obesity Assessment
4.5. Specimen Collection
4.6. Routine Laboratory Biochemical Analysis
4.6.1. Plasma Glucose Assessment
4.6.2. Glycated Hemoglobin Assessment
4.6.3. Serum Insulin Assessment
4.6.4. Homeostatic Model Assessment for Insulin Resistance
4.6.5. High-Sensitivity C-Reactive Protein Assessment
4.6.6. Lipid Profile Assessment
4.6.7. Lipoprotein Assessment
4.6.8. Enzyme-Linked Immunosorbent Assay of Omega-6 Fatty Acids and Desaturase Enzyme Levels
4.6.9. Serum Omega-3 Fatty Acid Measurements
4.6.10. Estimation of Desaturase and Elongase Enzyme Activity for Omega-3 and -6 Pathways
4.7. Genomic Examination
4.8. DNA Sequencing
4.9. Sample Size Calculation
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix B
Appendix C
References
- Onyango, E.M.; Onyango, B.M. The Rise of Noncommunicable Diseases in Kenya: An Examination of the Time Trends and Contribution of the Changes in Diet and Physical Inactivity. J. Epidemiol. Glob. Health 2018, 8, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Numan, A.T.; Jawad, N.K.; Fawzi, H.A. Biochemical study of the risk of diabetes, prediabetic and insulin resistance in car painters and its association with mercury exposure: A retrospective case-control study. Toxicol. Res. 2024, 13, tfae221. [Google Scholar] [CrossRef] [PubMed]
- Alotaibi, A.; Perry, L.; Gholizadeh, L.; Al-Ganmi, A. Incidence and prevalence rates of diabetes mellitus in Saudi Arabia: An overview. J. Epidemiol. Glob. Health 2017, 7, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Mansour, A.A.; Al-Maliky, A.A.; Kasem, B.; Jabar, A.; Mosbeh, K.A. Prevalence of diagnosed and undiagnosed diabetes mellitus in adults aged 19 years and older in Basrah, Iraq. Diabetes Metab. Syndr. Obes. Targets Ther. 2014, 7, 139–144. [Google Scholar] [CrossRef]
- Pengpid, S.; Peltzer, K. Prevalence and factors associated with undiagnosed type 2 diabetes among adults in Iraq: Analysis of cross-sectional data from the 2015 STEPS survey. BMJ Open 2022, 12, e064293. [Google Scholar] [CrossRef]
- Balić, A.; Vlašić, D.; Žužul, K.; Marinović, B.; Bukvić Mokos, Z. Omega-3 Versus Omega-6 Polyunsaturated Fatty Acids in the Prevention and Treatment of Inflammatory Skin Diseases. Int. J. Mol. Sci. 2020, 21, 741. [Google Scholar] [CrossRef]
- Ojha, P.K.; Poudel, D.K.; Rokaya, A.; Maharjan, S.; Timsina, S.; Poudel, A.; Satyal, R.; Satyal, P.; Setzer, W.N. Chemical Compositions and Essential Fatty Acid Analysis of Selected Vegetable Oils and Fats. Compounds 2024, 4, 37–70. [Google Scholar] [CrossRef]
- Sanchez, E.L.; Lagunoff, M. Viral activation of cellular metabolism. Virology 2015, 479–480, 609–618. [Google Scholar] [CrossRef]
- Clarke, S.D.; Nakamura, M.T. Fatty Acid Structure and Synthesis. In Encyclopedia of Biological Chemistry, 2nd ed.; Lennarz, W.J., Lane, M.D., Eds.; Academic Press: Waltham, MA, USA, 2013; pp. 285–289. [Google Scholar]
- Calder, P.C. Long-chain polyunsaturated fatty acids and inflammation. Scand. J. Food Nutr. 2006, 50, 54–61. [Google Scholar] [CrossRef]
- Fekete, K.; Györei, E.; Lohner, S.; Verduci, E.; Agostoni, C.; Decsi, T. Long-chain polyunsaturated fatty acid status in obesity: A systematic review and meta-analysis. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2015, 16, 488–497. [Google Scholar] [CrossRef]
- Poreba, M.; Rostoff, P.; Siniarski, A.; Mostowik, M.; Golebiowska-Wiatrak, R.; Nessler, J.; Undas, A.; Gajos, G. Relationship between polyunsaturated fatty acid composition in serum phospholipids, systemic low-grade inflammation, and glycemic control in patients with type 2 diabetes and atherosclerotic cardiovascular disease. Cardiovasc. Diabetol. 2018, 17, 29. [Google Scholar] [CrossRef]
- Petersen, K.S.; Maki, K.C.; Calder, P.C.; Belury, M.A.; Messina, M.; Kirkpatrick, C.F.; Harris, W.S. Perspective on the health effects of unsaturated fatty acids and commonly consumed plant oils high in unsaturated fat. Br. J. Nutr. 2024, 132, 1039–1050. [Google Scholar] [CrossRef] [PubMed]
- Kothri, M.; Mavrommati, M.; Elazzazy, A.M.; Baeshen, M.N.; Moussa, T.A.A.; Aggelis, G. Microbial sources of polyunsaturated fatty acids (PUFAs) and the prospect of organic residues and wastes as growth media for PUFA-producing microorganisms. FEMS Microbiol. Lett. 2020, 367, fnaa028. [Google Scholar] [CrossRef] [PubMed]
- Czumaj, A.; Śledziński, T. Biological Role of Unsaturated Fatty Acid Desaturases in Health and Disease. Nutrients 2020, 12, 356. [Google Scholar] [CrossRef] [PubMed]
- Korbecki, J.; Rębacz-Maron, E.; Kupnicka, P.; Chlubek, D.; Baranowska-Bosiacka, I. Synthesis and Significance of Arachidonic Acid, a Substrate for Cyclooxygenases, Lipoxygenases, and Cytochrome P450 Pathways in the Tumorigenesis of Glioblastoma Multiforme, Including a Pan-Cancer Comparative Analysis. Cancers 2023, 15, 946. [Google Scholar] [CrossRef]
- Kytikova, O.Y.; Novgorodtseva, T.P.; Antonyuk, M.; Gvozdenko, T.A. Associations of delta fatty acid desaturase gene polymorphisms with lipid metabolism disorders. Russ. Open Med. J. 2021, 10, 403. [Google Scholar] [CrossRef]
- Monroig, Ó.; Shu-Chien, A.C.; Kabeya, N.; Tocher, D.R.; Castro, L.F.C. Desaturases and elongases involved in long-chain polyunsaturated fatty acid biosynthesis in aquatic animals: From genes to functions. Prog. Lipid Res. 2022, 86, 101157. [Google Scholar] [CrossRef]
- O’Neill, L.M.; Miyazaki, M.; Bond, L.M.; Lewis, S.A.; Ding, F.; Liu, Z.; Ntambi, J.M. Chapter 6-Fatty acid desaturation and elongation in mammals. In Biochemistry of Lipids, Lipoproteins and Membranes, 7th ed.; Ridgway, N.D., McLeod, R.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 201–226. [Google Scholar]
- Howard, T.D.; Mathias, R.A.; Seeds, M.C.; Herrington, D.M.; Hixson, J.E.; Shimmin, L.C.; Hawkins, G.A.; Sellers, M.; Ainsworth, H.C.; Sergeant, S.; et al. DNA methylation in an enhancer region of the FADS cluster is associated with FADS activity in human liver. PLoS ONE 2014, 9, e97510. [Google Scholar] [CrossRef]
- Žák, A.; Jáchymová, M.; Burda, M.; Staňková, B.; Zeman, M.; Slabý, A.; Vecka, M.; Šeda, O. FADS Polymorphisms Affect the Clinical and Biochemical Phenotypes of Metabolic Syndrome. Metabolites 2022, 12, 568. [Google Scholar] [CrossRef]
- Jojima, K.; Kihara, A. Metabolism of sphingadiene and characterization of the sphingadiene-producing enzyme FADS3. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2023, 1868, 159335. [Google Scholar] [CrossRef]
- Kaptoge, S.; Seshasai, S.R.K.; Sun, L.; Walker, M.; Bolton, T.; Spackman, S.; Ataklte, F.; Willeit, P.; Bell, S.; Burgess, S.; et al. Life expectancy associated with different ages at diagnosis of type 2 diabetes in high-income countries: 23 million person-years of observation. Lancet Diabetes Endocrinol. 2023, 11, 731–742. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-J.; Feng, J.; Zhang, X.-T.; Zhang, H.-Z. Age at type 2 diabetes diagnosis and the risk of mortality among US population. Sci. Rep. 2024, 14, 29155. [Google Scholar] [CrossRef]
- Yao, X.; Zhang, J.; Zhang, X.; Jiang, T.; Zhang, Y.; Dai, F.; Hu, H.; Zhang, Q. Age at diagnosis, diabetes duration and the risk of cardiovascular disease in patients with diabetes mellitus: A cross-sectional study. Front. Endocrinol. 2023, 14, 1131395. [Google Scholar] [CrossRef]
- Hamid, S.; Sahib, H.B. Age and General Characteristic Effect on Iraqi Patients with Type 2 Diabetes Mellitus. IOP Conf. Ser. Mater. Sci. Eng. 2018, 454, 012018. [Google Scholar] [CrossRef]
- Mohammed, M.S.; Ahmed, H.S. Atherogenic indices in type 2 diabetic Iraqi patients and its association with cardiovascular disease risk. J. Fac. Med. Baghdad 2023, 65, 179–186. [Google Scholar]
- Mohammed, H.R.; Kadhim, K.A.; Alkutubi, H.M.; Rahmah, A.M.; Khalaf, B.H.; Hussein, S.A.-R.; Fawzi, H.A. Correlation between ABO blood groups with insulin resistance in type II diabetes mellitus patients using Metformin. Int. J. Res. Pharm. Sci. 2018, 9, 893–900. [Google Scholar]
- Hamid, S.; Sahib, H.B.; Fawzi, H.A.; Nori, A.Y. Medication adherence and glycemic control in newly diagnosed diabetic patients. Int. J. Res. Pharm. Sci. 2018, 9, 816–820. [Google Scholar]
- Atia, Y.A.; Mohammed, S.T.; Abdullah, S.S.; Abbas, A.S.; Fawzi, H.A. Effects of subclinical hypothyroidism in type II diabetes mellitus patients on biochemical, coagulation, and fibrinolysis status. J. Adv. Pharm. Technol. Res. 2024, 15, 130–134. [Google Scholar] [CrossRef]
- Khudair, F.A.; Ali, S.H.; Al-Nuaimi, A.A.M. Study of Association between RAGE Gene Polymorphism rs2070600 (G82S) and Aspirin Resistance in Coronary Artery Disease Iraqi Patients with and without Type 2 Diabetes. J. Pharm. Negat. Results 2022, 13, 170–182. [Google Scholar]
- Wang, L.; Huang, X.; Sun, M.; Zheng, T.; Zheng, L.; Lin, X.; Ruan, J.; Lin, F. New light on ω-3 polyunsaturated fatty acids and diabetes debate: A population pharmacokinetic-pharmacodynamic modelling and intake threshold study. Nutr. Diabetes 2024, 14, 8. [Google Scholar] [CrossRef]
- Kazemian, P.; Kazemi-Bajestani, S.M.; Alherbish, A.; Steed, J.; Oudit, G.Y. The use of ω-3 poly-unsaturated fatty acids in heart failure: A preferential role in patients with diabetes. Cardiovasc. Drugs Ther 2012, 26, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Delpino, F.M.; Figueiredo, L.M.; da Silva, B.G.C.; da Silva, T.G.; Mintem, G.C.; Bielemann, R.M.; Gigante, D.P. Omega-3 supplementation and diabetes: A systematic review and meta-analysis. Crit. Rev. Food Sci. Nutr. 2022, 62, 4435–4448. [Google Scholar] [CrossRef] [PubMed]
- Gortan Cappellari, G.; Semolic, A.; Ruozi, G.; Barbetta, D.; Bortolotti, F.; Vinci, P.; Zanetti, M.; Mak, R.H.; Garibotto, G.; Giacca, M.; et al. n-3 PUFA dietary lipid replacement normalizes muscle mitochondrial function and oxidative stress through enhanced tissue mitophagy and protects from muscle wasting in experimental kidney disease. Metabolism 2022, 133, 155242. [Google Scholar] [CrossRef]
- Durán, A.M.; Beeson, W.L.; Firek, A.; Cordero-MacIntyre, Z.; De León, M. Dietary Omega-3 Polyunsaturated Fatty-Acid Supplementation Upregulates Protective Cellular Pathways in Patients with Type 2 Diabetes Exhibiting Improvement in Painful Diabetic Neuropathy. Nutrients 2022, 14, 761. [Google Scholar] [CrossRef]
- Lemaitre, R.N.; Tanaka, T.; Tang, W.; Manichaikul, A.; Foy, M.; Kabagambe, E.K.; Nettleton, J.A.; King, I.B.; Weng, L.C.; Bhattacharya, S.; et al. Genetic loci associated with plasma phospholipid n-3 fatty acids: A meta-analysis of genome-wide association studies from the CHARGE Consortium. PLoS Genet. 2011, 7, e1002193. [Google Scholar] [CrossRef]
- (rs174547) F. rs17454. dsSPN. 2025. Available online: https://www.ncbi.nlm.nih.gov/snp/rs174547 (accessed on 15 February 2025).
- Huang, M.C.; Chang, W.T.; Chang, H.Y.; Chung, H.F.; Chen, F.P.; Huang, Y.F.; Hsu, C.C.; Hwang, S.J. FADS Gene Polymorphisms, Fatty Acid Desaturase Activities, and HDL-C in Type 2 Diabetes. Int. J. Environ. Res. Public Health 2017, 14, 572. [Google Scholar] [CrossRef] [PubMed]
- Niwa, S.; Kawabata, T.; Shoji, K.; Ogata, H.; Kagawa, Y.; Nakayama, K.; Yanagisawa, Y.; Iwamoto, S.; Tatsuta, N.; Asato, K.; et al. Investigation of Maternal Diet and FADS1 Polymorphism Associated with Long-Chain Polyunsaturated Fatty Acid Compositions in Human Milk. Nutrients 2022, 14, 2160. [Google Scholar] [CrossRef]
- Coltell, O.; Asensio, E.M.; Sorlí, J.V.; Barragán, R.; Fernández-Carrión, R.; Portolés, O.; Ortega-Azorín, C.; Martínez-LaCruz, R.; González, J.I.; Zanón-Moreno, V.; et al. Genome-Wide Association Study (GWAS) on Bilirubin Concentrations in Subjects with Metabolic Syndrome: Sex-Specific GWAS Analysis and Gene-Diet Interactions in a Mediterranean Population. Nutrients 2019, 11, 90. [Google Scholar] [CrossRef]
- Coltell, O.; Sorlí, J.V.; Asensio, E.M.; Barragán, R.; González, J.I.; Giménez-Alba, I.M.; Zanón-Moreno, V.; Estruch, R.; Ramírez-Sabio, J.B.; Pascual, E.C.; et al. Genome-Wide Association Study for Serum Omega-3 and Omega-6 Polyunsaturated Fatty Acids: Exploratory Analysis of the Sex-Specific Effects and Dietary Modulation in Mediterranean Subjects with Metabolic Syndrome. Nutrients 2020, 12, 310. [Google Scholar] [CrossRef]
- Park, S.; Kim, D.S.; Kang, S. Carrying minor allele of FADS1 and haplotype of FADS1 and FADS2 increased the risk of metabolic syndrome and moderate but not low fat diets lowered the risk in two Korean cohorts. Eur. J. Nutr. 2019, 58, 831–842. [Google Scholar] [CrossRef]
- Ching, Y.K.; Chin, Y.S.; Appukutty, M.; Ramanchadran, V.; Yu, C.Y.; Ang, G.Y.; Gan, W.Y.; Chan, Y.M.; Teh, L.K.; Salleh, M.Z. Interaction of Dietary Linoleic Acid and α-Linolenic Acids with rs174547 in FADS1 Gene on Metabolic Syndrome Components among Vegetarians. Nutrients 2019, 11, 1686. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Yang, Y.; Yu, X.; Hu, S.; Shao, S. Association between omega-3 fatty acids consumption and the risk of type 2 diabetes: A meta-analysis of cohort studies. J. Diabetes Investig. 2017, 8, 480–488. [Google Scholar] [CrossRef]
- Djuricic, I.; Calder, P.C. Polyunsaturated fatty acids and metabolic health: Novel insights. Curr. Opin. Clin. Nutr. Metab. Care 2022, 25, 436–442. [Google Scholar] [CrossRef] [PubMed]
- The ASCEND Study Collaborative Group. Effects of n−3 Fatty Acid Supplements in Diabetes Mellitus. N. Engl. J. Med. 2018, 379, 1540–1550. [Google Scholar] [CrossRef]
- Vessby, B.; Karlström, B.; Boberg, M.; Lithell, H.; Berne, C. Polyunsaturated fatty acids may impair blood glucose control in type 2 diabetic patients. Diabet. Med. 1992, 9, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Glauber, H.; Wallace, P.; Griver, K.; Brechtel, G. Adverse metabolic effect of omega-3 fatty acids in non-insulin-dependent diabetes mellitus. Ann. Intern. Med. 1988, 108, 663–668. [Google Scholar] [CrossRef]
- Jäger, S.; Cuadrat, R.; Hoffmann, P.; Wittenbecher, C.; Schulze, M.B. Desaturase Activity and the Risk of Type 2 Diabetes and Coronary Artery Disease: A Mendelian Randomization Study. Nutrients 2020, 12, 2261. [Google Scholar] [CrossRef]
- Huang, R.; Yan, L.; Lei, Y. The relationship between high-density lipoprotein cholesterol (HDL-C) and glycosylated hemoglobin in diabetic patients aged 20 or above: A cross-sectional study. BMC Endocr. Disord. 2021, 21, 198. [Google Scholar] [CrossRef]
- Sacks, F.M.; Andraski, A.B. Dietary fat and carbohydrate affect the metabolism of protein-based high-density lipoprotein subspecies. Curr. Opin. Lipidol. 2022, 33, 1–15. [Google Scholar] [CrossRef]
- Li, M.; Liu, Y.; Li, Q.; Yang, M.; Pi, Y.; Yang, N.; Zheng, Y.; Yue, X. Comparative exploration of free fatty acids in donkey colostrum and mature milk based on a metabolomics approach. J. Dairy Sci. 2020, 103, 6022–6031. [Google Scholar] [CrossRef]
- Fillmore, N.; Mori, J.; Lopaschuk, G.D. Mitochondrial fatty acid oxidation alterations in heart failure, ischaemic heart disease and diabetic cardiomyopathy. Br. J. Pharmacol. 2014, 171, 2080–2090. [Google Scholar] [CrossRef] [PubMed]
- Bentsen, H. Dietary polyunsaturated fatty acids, brain function and mental health. Microb. Ecol. Health Dis. 2017, 28, 1281916. [Google Scholar] [CrossRef]
- Ruiz, M.; Bodhicharla, R.; Svensk, E.; Devkota, R.; Busayavalasa, K.; Palmgren, H.; Ståhlman, M.; Boren, J.; Pilon, M. Membrane fluidity is regulated by the C. elegans transmembrane protein FLD-1 and its human homologs TLCD1/2. Elife 2018, 7, e40686. [Google Scholar] [CrossRef]
- Mititelu, M.; Lupuliasa, D.; Neacșu, S.M.; Olteanu, G.; Busnatu, Ș.S.; Mihai, A.; Popovici, V.; Măru, N.; Boroghină, S.C.; Mihai, S.; et al. Polyunsaturated Fatty Acids and Human Health: A Key to Modern Nutritional Balance in Association with Polyphenolic Compounds from Food Sources. Foods 2025, 14, 46. [Google Scholar] [CrossRef]
- Lepretti, M.; Martucciello, S.; Burgos Aceves, M.A.; Putti, R.; Lionetti, L. Omega-3 Fatty Acids and Insulin Resistance: Focus on the Regulation of Mitochondria and Endoplasmic Reticulum Stress. Nutrients 2018, 10, 350. [Google Scholar] [CrossRef] [PubMed]
- Shearer, G.C.; Savinova, O.V.; Harris, W.S. Fish oil—How does it reduce plasma triglycerides? Biochim. Biophys. Acta 2012, 1821, 843–851. [Google Scholar] [CrossRef] [PubMed]
- Shimano, H.; Sato, R. SREBP-regulated lipid metabolism: Convergent physiology-divergent pathophysiology. Nat. Rev. Endocrinol. 2017, 13, 710–730. [Google Scholar] [CrossRef]
- Backes, J.; Anzalone, D.; Hilleman, D.; Catini, J. The clinical relevance of omega-3 fatty acids in the management of hypertriglyceridemia. Lipids Health Dis. 2016, 15, 118. [Google Scholar] [CrossRef]
- Wang, M.; Ma, L.J.; Yang, Y.; Xiao, Z.; Wan, J.B. n-3 Polyunsaturated fatty acids for the management of alcoholic liver disease: A critical review. Crit. Rev. Food Sci. Nutr. 2019, 59, S116–S129. [Google Scholar] [CrossRef]
- Wang, H.; Maechler, P.; Antinozzi, P.A.; Herrero, L.; Hagenfeldt-Johansson, K.A.; Bjorklund, A.; Wollheim, C.B. The transcription factor SREBP-1c is instrumental in the development of beta-cell dysfunction. J. Biol. Chem. 2003, 278, 16622–16629. [Google Scholar] [CrossRef]
- Kalupahana, N.S.; Claycombe, K.J.; Moustaid-Moussa, N. (n-3) Fatty acids alleviate adipose tissue inflammation and insulin resistance: Mechanistic insights. Adv. Nutr. 2011, 2, 304–316. [Google Scholar] [CrossRef] [PubMed]
- Djuricic, I.; Calder, P.C. Beneficial Outcomes of Omega-6 and Omega-3 Polyunsaturated Fatty Acids on Human Health: An Update for 2021. Nutrients 2021, 13, 2421. [Google Scholar] [CrossRef]
- Sjögren, P.; Sierra-Johnson, J.; Gertow, K.; Rosell, M.; Vessby, B.; de Faire, U.; Hamsten, A.; Hellenius, M.L.; Fisher, R.M. Fatty acid desaturases in human adipose tissue: Relationships between gene expression, desaturation indexes and insulin resistance. Diabetologia 2008, 51, 328–335. [Google Scholar] [CrossRef]
- Merino, D.M.; Ma, D.W.; Mutch, D.M. Genetic variation in lipid desaturases and its impact on the development of human disease. Lipids Health Dis. 2010, 9, 63. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, H.; Sueyasu, T.; Tokuda, H.; Ito, M.; Kaneda, Y.; Rogi, T.; Kawashima, H.; Horiguchi, S.; Kawabata, T.; Shibata, H. Aging and FADS1 polymorphisms decrease the biosynthetic capacity of long-chain PUFAs: A human trial using [U-(13)C]linoleic acid. Prostaglandins Leukot. Essent. Fat. Acids 2019, 148, 1–8. [Google Scholar] [CrossRef]
- Muzsik, A.; Bajerska, J.; Jeleń, H.H.; Gaca, A.; Chmurzynska, A. Associations between Fatty Acid Intake and Status, Desaturase Activities, and FADS Gene Polymorphism in Centrally Obese Postmenopausal Polish Women. Nutrients 2018, 10, 1068. [Google Scholar] [CrossRef]
- Guan, W.; Steffen, B.T.; Lemaitre, R.N.; Wu, J.H.Y.; Tanaka, T.; Manichaikul, A.; Foy, M.; Rich, S.S.; Wang, L.; Nettleton, J.A.; et al. Genome-wide association study of plasma N6 polyunsaturated fatty acids within the cohorts for heart and aging research in genomic epidemiology consortium. Circ. Cardiovasc. Genet. 2014, 7, 321–331. [Google Scholar] [CrossRef]
- Plunde, O.; Larsson, S.C.; Artiach, G.; Thanassoulis, G.; Carracedo, M.; Franco-Cereceda, A.; Eriksson, P.; Bäck, M. FADS1 (Fatty Acid Desaturase 1) Genotype Associates with Aortic Valve FADS mRNA Expression, Fatty Acid Content and Calcification. Circ. Genom. Precis. Med. 2020, 13, e002710. [Google Scholar] [CrossRef] [PubMed]
- Rabehl, M.; Wei, Z.; Leineweber, C.G.; Enssle, J.; Rothe, M.; Jung, A.; Schmöcker, C.; Elbelt, U.; Weylandt, K.H.; Pietzner, A. Effect of FADS1 SNPs rs174546, rs174547 and rs174550 on blood fatty acid profiles and plasma free oxylipins. Front. Nutr. 2024, 2024, 1356986. [Google Scholar] [CrossRef]
- Kröger, J.; Zietemann, V.; Enzenbach, C.; Weikert, C.; Jansen, E.H.; Döring, F.; Joost, H.G.; Boeing, H.; Schulze, M.B. Erythrocyte membrane phospholipid fatty acids, desaturase activity, and dietary fatty acids in relation to risk of type 2 diabetes in the European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Am. J. Clin. Nutr. 2011, 93, 127–142. [Google Scholar] [CrossRef]
- Vessby, B.; Gustafsson, I.B.; Tengblad, S.; Boberg, M.; Andersson, A. Desaturation and elongation of fatty acids and insulin action. Ann. N. Y. Acad. Sci. 2002, 967, 183–195. [Google Scholar] [CrossRef]
- Risérus, U. Fatty acids and insulin sensitivity. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Corpeleijn, E.; Feskens, E.J.M.; Jansen, E.H.J.M.; Mensink, M.; Saris, W.H.M.; De Bruin, T.W.A.; Blaak, E.E. Improvements in glucose tolerance and insulin sensitivity after lifestyle intervention are related to changes in serum fatty acid profile and desaturase activities: The SLIM study. Diabetologia 2006, 49, 2392–2401. [Google Scholar] [CrossRef][Green Version]
- Ingelsson, E.; Langenberg, C.; Hivert, M.F.; Prokopenko, I.; Lyssenko, V.; Dupuis, J.; Mägi, R.; Sharp, S.; Jackson, A.U.; Assimes, T.L.; et al. Detailed physiologic characterization reveals diverse mechanisms for novel genetic loci regulating glucose and insulin metabolism in humans. Diabetes 2010, 59, 1266–1275. [Google Scholar] [CrossRef] [PubMed]
- Boesgaard, T.W.; Grarup, N.; Jørgensen, T.; Borch-Johnsen, K.; Hansen, T.; Pedersen, O. Variants at DGKB/TMEM195, ADRA2A, GLIS3 and C2CD4B loci are associated with reduced glucose-stimulated beta cell function in middle-aged Danish people. Diabetologia 2010, 53, 1647–1655. [Google Scholar] [CrossRef] [PubMed]
- Risérus, U.; Willett, W.C.; Hu, F.B. Dietary fats and prevention of type 2 diabetes. Prog. Lipid Res. 2009, 48, 44–51. [Google Scholar] [CrossRef]
- Manco, M.; Calvani, M.; Mingrone, G. Effects of dietary fatty acids on insulin sensitivity and secretion. Diabetes Obes. Metab. 2004, 6, 402–413. [Google Scholar] [CrossRef]
- Galgani, J.E.; Uauy, R.D.; Aguirre, C.A.; Díaz, E.O. Effect of the dietary fat quality on insulin sensitivity. Br. J. Nutr. 2008, 100, 471–479. [Google Scholar] [CrossRef]
- Hwalla, N.; Jaafar, Z.; Sawaya, S. Dietary Management of Type 2 Diabetes in the MENA Region: A Review of the Evidence. Nutrients 2021, 13, 1060. [Google Scholar] [CrossRef]
- Committee ADAPP. 2. Diagnosis and Classification of Diabetes: Standards of Care in Diabetes—2024. Diabetes Care 2023, 47 (Suppl. 1), S20–S42. [Google Scholar]
- Cuschieri, S. The STROBE guidelines. Saudi J. Anaesth. 2019, 13 (Suppl. 1), S31–S34. [Google Scholar] [CrossRef] [PubMed]
- Little, J.; Higgins, J.P.; Ioannidis, J.P.; Moher, D.; Gagnon, F.; von Elm, E.; Khoury, M.J.; Cohen, B.; Davey-Smith, G.; Grimshaw, J.; et al. STrengthening the REporting of Genetic Association Studies (STREGA)—An extension of the STROBE statement. Genet. Epidemiol. 2009, 33, 581–598. [Google Scholar] [CrossRef] [PubMed]
- Devajit, M.; Haradhan Kumar, M. Body Mass Index (BMI) is a Popular Anthropometric Tool to Measure Obesity Among Adults. J. Innov. Med. Res. 2023, 2, 25–33. [Google Scholar]
- NCD Risk Factor Collaboration. Trends in adult body-mass index in 200 countries from 1975 to 2014: A pooled analysis of 1698 population-based measurement studies with 19·2 million participants. Lancet 2016, 387, 1377–1396. [Google Scholar] [CrossRef] [PubMed]
- Umuerri, E.M. Chapter 17-Ethnicity and Cut-Off Values in Obesity. In Nutrition in the Prevention and Treatment of Abdominal Obesity, 2nd ed.; Watson, R.R., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 211–223. [Google Scholar]
- WHO Expert Consultation. Waist Circumference and Waist-Hip Ratio; Report of a WHO Expert Consultation; World Health Organization: Geneva, Switzerland, 2008; pp. 8–11. [Google Scholar]
- Numan, A.T.; Jawad, N.K.; Fawzi, H.A. Biochemical study of the effect of lead exposure in nonobese gasoline station workers and risk of hyperglycemia: A retrospective case-control study. Medicine 2024, 103, e39152. [Google Scholar] [CrossRef]
- Sonagra, A.D.; Zubair, M.; Motiani, A. Hexokinase Method. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Klingenberg, O.; Furuset, T.; Hestbråten, C.R.; Hallberg, M.H.; Steiro, A.; Orset, I.R.; Berg, J.P. HbA1c analysis by capillary electrophoresis-comparison with chromatography and an immunological method. Scand. J. Clin. Lab. Investig. 2017, 77, 458–464. [Google Scholar] [CrossRef]
- Mahadevarao Premnath, S.; Zubair, M. Electrochemiluminescence Method. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Sapin, R.; Le Galudec, V.; Gasser, F.; Pinget, M.; Grucker, D. Elecsys insulin assay: Free insulin determination and the absence of cross-reactivity with insulin lispro. Clin. Chem. 2001, 47, 602–605. [Google Scholar] [CrossRef]
- Dasgupta, R.; Shetty, S.P. Chapter 29—Assessment of insulin resistance: From the bench to bedside. In Metabolic Syndrome; Mukhopadhyay, S., Mondal, S., Eds.; Academic Press: Cambridge, MA, USA, 2024; pp. 351–365. [Google Scholar]
- Price, C.P.; Trull, A.K.; Berry, D.; Gorman, E.G. Development and validation of a particle-enhanced turbidimetric immunoassay for C-reactive protein. J. Immunol. Methods 1987, 99, 205–211. [Google Scholar] [CrossRef]
- Bucolo, G.; David, H. Quantitative determination of serum triglycerides by the use of enzymes. Clin. Chem. 1973, 19, 476–482. [Google Scholar] [CrossRef]
- Allain, C.C.; Poon, L.S.; Chan, C.S.; Richmond, W.; Fu, P.C. Enzymatic determination of total serum cholesterol. Clin. Chem. 1974, 20, 470–475. [Google Scholar] [CrossRef]
- Nauck, M.; Graziani, M.S.; Jarausch, J.; Bruton, D.; Cobbaert, C.; Cole, T.G.; Colella, F.; Lefevre, F.; Gillery, P.; Haas, B.; et al. A new liquid homogeneous assay for HDL cholesterol determination evaluated in seven laboratories in Europe and the United States. Clin. Chem. Lab. Med. 1999, 37, 1067–1076. [Google Scholar] [CrossRef] [PubMed]
- Rifai, N.; Warnick, G.R.; McNamara, J.R.; Belcher, J.D.; Grinstead, G.F.; Frantz, I.D., Jr. Measurement of low-density-lipoprotein cholesterol in serum: A status report. Clin. Chem. 1992, 38, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Siedel, J.; Schmuck, R.; Staepels, J.; Town, M. Long term stable, liquid ready-to-use monoreagent for the enzymatic assay of serum or plasma triglycerides (GPO-PAP method). AACC meeting abstract 34. Clin. Chem. 1993, 39, 1127. [Google Scholar]
- Pisani, T.; Gebski, C.P.; Leary, E.T.; Warnick, G.R.; Ollington, J.F. Accurate direct determination of low-density lipoprotein cholesterol using an immunoseparation reagent and enzymatic cholesterol assay. Arch. Pathol. Lab. Med. 1995, 119, 1127–1135. [Google Scholar]
- Miida, T.; Nishimura, K.; Okamura, T.; Hirayama, S.; Ohmura, H.; Yoshida, H.; Miyashita, Y.; Ai, M.; Tanaka, A.; Sumino, H.; et al. Validation of homogeneous assays for HDL-cholesterol using fresh samples from healthy and diseased subjects. Atherosclerosis 2014, 233, 253–259. [Google Scholar] [CrossRef]
- Tighe, D.A.; Ockene, I.S.; Reed, G.; Nicolosi, R. Calculated low density lipoprotein cholesterol levels frequently underestimate directly measured low density lipoprotein cholesterol determinations in patients with serum triglyceride levels ≤ 4.52 mmol/L: An analysis comparing the LipiDirect® magnetic LDL assay with the Friedewald calculation. Clin. Chim. Acta 2006, 365, 236–242. [Google Scholar]
- Karvane, H.B.; Esfandiari, H.; Qutaiba, O.; Allela, B.; Mahdi, M.S.; Al-Nuaimi, A.M.A.; Al-hussein, R.K.A.; Jawad, M.J. Metabolic syndrome in association with novel dietary index, metabolic parameters, nesfatin-1 and omentin-1. BMC Endocr. Disord. 2024, 24, 257. [Google Scholar] [CrossRef]
- Islam, S.M.T.; Osa-Andrews, B.; Jones, P.M.; Muthukumar, A.R.; Hashim, I.; Cao, J. Methods of Low-Density Lipoprotein-Cholesterol Measurement: Analytical and Clinical Applications. Ejifcc 2022, 33, 282–294. [Google Scholar]
- Siedel, J.; Schiefer, S.; Rosseneu, M.; Bergeaud, R.; De Keersgieter, W.; Pautz, B.; Vinaimont, N.; Ziegenhorn, J. Immunoturbidimetric method for routine determinations of apolipoproteins A-I, A-II, and B in normo- and hyperlipemic sera compared with immunonephelometry. Clin. Chem. 1988, 34, 1821–1825. [Google Scholar] [CrossRef]
- Rifai, N.; King, M.E. Immunoturbidimetric assays of apolipoproteins A, AI, AII, and B in serum. Clin. Chem. 1986, 32, 957–961. [Google Scholar] [CrossRef]
- Zurek, G.; Gee, S.J.; Hammock, B.D. Development of an enzyme immunoassay for linoleic acid diols in urine. Anal. Chim. Acta 2002, 466, 247–256. [Google Scholar] [CrossRef]
- Chu, X.; Ageishi, Y.; Nishimura, K.; Jisaka, M.; Nagaya, T.; Shono, F.; Yokota, K. Development of enzyme-linked immunosorbent assay for 8-iso-prostaglandin F2alpha, a biomarker of oxidative stress in vivo, and its application to the quantification in aged rats. J. Pharm. Biomed. Anal. 2009, 50, 911–916. [Google Scholar] [CrossRef] [PubMed]
- Balzer, A.H.A.; Whitehurst, C.B. An Analysis of the Biotin-(Strept)avidin System in Immunoassays: Interference and Mitigation Strategies. Curr. Issues Mol. Biol. 2023, 45, 8733–8754. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Sun, L.; Sheng, J.; Jin, Z.; Sun, Y.; Wang, S.; Su, P.; Hao, J.; Tao, F. Determination of fatty acids in maternal serum by gas chromatography and mass spectrometry to evaluate the association with mental retardation in children. J. Chem. Pharm. Res. 2015, 7, 525–533. [Google Scholar]
- Li, S.W.; Wang, J.; Yang, Y.; Liu, Z.J.; Cheng, L.; Liu, H.Y.; Ma, P.; Luo, W.; Liu, S.M. Polymorphisms in FADS1 and FADS2 alter plasma fatty acids and desaturase levels in type 2 diabetic patients with coronary artery disease. J. Transl. Med. 2016, 14, 79. [Google Scholar] [CrossRef]
- Domínguez-López, I.; Arancibia-Riveros, C.; Tresserra-Rimbau, A.; Castro-Barquero, S.; Casas, R.; Vázquez-Ruiz, Z.; Ros, E.; Fitó, M.; Estruch, R.; López-Sabater, M.C.; et al. Relationship between estimated desaturase enzyme activity and metabolic syndrome in a longitudinal study. Front. Nutr. 2022, 9, 991277. [Google Scholar] [CrossRef]
- Chacon-Cortes, D.; Griffiths, L.R. Methods for extracting genomic DNA from whole blood samples: Current perspectives. J. Biorepository Sci. Appl. Med. 2014, 2, 1–9. [Google Scholar]
- Zaid, A.; Al-Kazaz, A.A. Genetic polymorphisms of crisp2 gene in association with infertility in Iraqi patients. Iraqi J. Agric. Sci. 2023, 54, 657–666. [Google Scholar] [CrossRef]
- Al-Radeef, M.Y.; Fawzi, H.A.; Allawi, A.A. ACE gene polymorphism and its association with serum erythropoietin and hemoglobin in Iraqi hemodialysis patients. Appl. Clin. Genet. 2019, 12, 107–112. [Google Scholar] [CrossRef]
- Harisha, S. Biotechnology Procedures and Experiments Handbook; Laxmi Publications, Ltd.: New Delhi, India, 2008. [Google Scholar]
- Al-Radeef, M.Y.; Allawi, A.A.D.; Fawzi, H.A. Interleukin-6 gene polymorphisms and serum erythropoietin and hemoglobin in hemodialysis Iraqi patients. Saudi J. Kidney Dis. Transplant. 2018, 29, 1042–1049. [Google Scholar] [CrossRef]
- Adams, D.S. Lab Math: A Handbook of Measurements, Calculations, and Other Quantitative Skills for Use at the Bench; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2003. [Google Scholar]
- Tataurov, A.V.; You, Y.; Owczarzy, R. Predicting ultraviolet spectrum of single stranded and double stranded deoxyribonucleic acids. Biophys. Chem. 2008, 133, 66–70. [Google Scholar] [CrossRef]
- Hegazi, M.A.; Seleem, A.; EL-Adawy, E.H.; Elhussini, M.E. Association of IGF-I gene polymorphism with diabetic nephropathy in Egyptians with type 2 diabetes. Egypt. J. Intern. Med. 2018, 30, 191–196. [Google Scholar] [CrossRef]
- Koressaar, T.; Remm, M. Enhancements and modifications of primer design program Primer3. Bioinformatics 2007, 23, 1289–1291. [Google Scholar] [CrossRef] [PubMed]
- Untergasser, A.; Cutcutache, I.; Koressaar, T.; Faircloth, B.C.; Remm, M.; Rozen, S.G. Primer3--new capabilities and interfaces. Nucleic Acids Res. 2012, 40, e115. [Google Scholar] [CrossRef]
- Karolchik, D.; Hinrichs, A.S.; Kent, W.J. The UCSC Genome Browser. Curr. Protoc. Hum. Genet. 2011, 18, 18.6.1–18.6.33. [Google Scholar] [CrossRef] [PubMed]
- Mu, M.; Yang, F.; Han, B.; Tian, X.; Zhang, K. Manure application: A trigger for vertical accumulation of antibiotic resistance genes in cropland soils. Ecotoxicol. Environ. Saf. 2022, 237, 113555. [Google Scholar] [CrossRef] [PubMed]
- Coleman, W.B.; Tsongalis, G.J. Molecular Diagnostics: For the Clinical Laboratorian; Springer Science & Business Media: Berlin, Germany, 2006. [Google Scholar]
- Sanger, F.; Nicklen, S.; Coulson, A.R. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA 1977, 74, 5463–5467. [Google Scholar] [CrossRef]
- Hall, T.; Biosciences, I.; Carlsbad, C. BioEdit: An important software for molecular biology. GERF Bull. Biosci. 2011, 2, 60–61. [Google Scholar]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.G. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef]
- Shen, J.; Li, Z.; Chen, J.; Song, Z.; Zhou, Z.; Shi, Y. SHEsisPlus, a toolset for genetic studies on polyploid species. Sci. Rep. 2016, 6, 24095. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.Y.; He, L. SHEsis, a powerful software platform for analyses of linkage disequilibrium, haplotype construction, and genetic association at polymorphism loci. Cell Res. 2005, 15, 97–98. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, Z.; He, Z.; Tang, W.; Li, T.; Zeng, Z.; He, L.; Shi, Y. A partition-ligation-combination-subdivision EM algorithm for haplotype inference with multiallelic markers: Update of the SHEsis (http://analysis.bio-x.cn). Cell Res. 2009, 19, 519–523. [Google Scholar] [CrossRef] [PubMed]

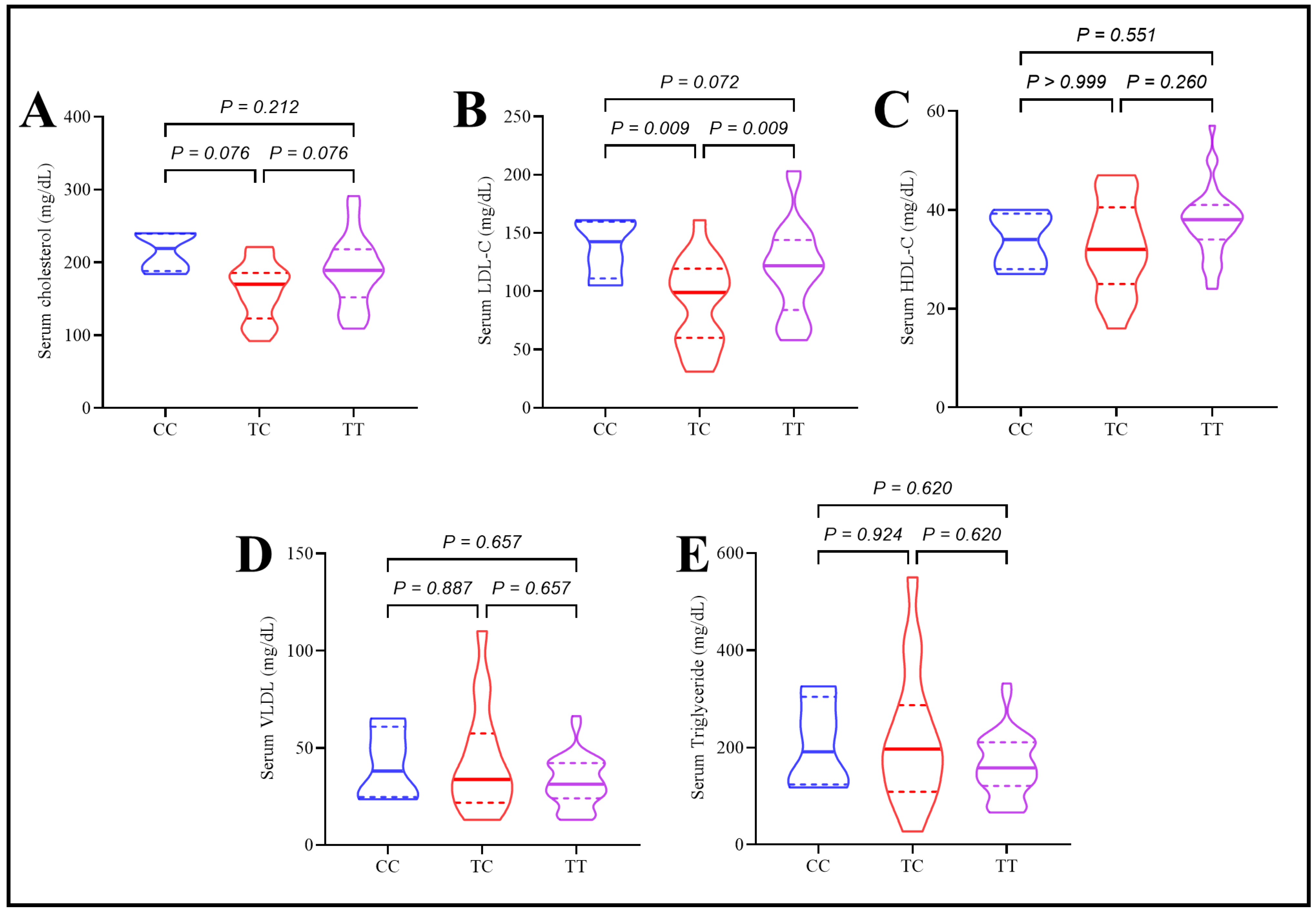
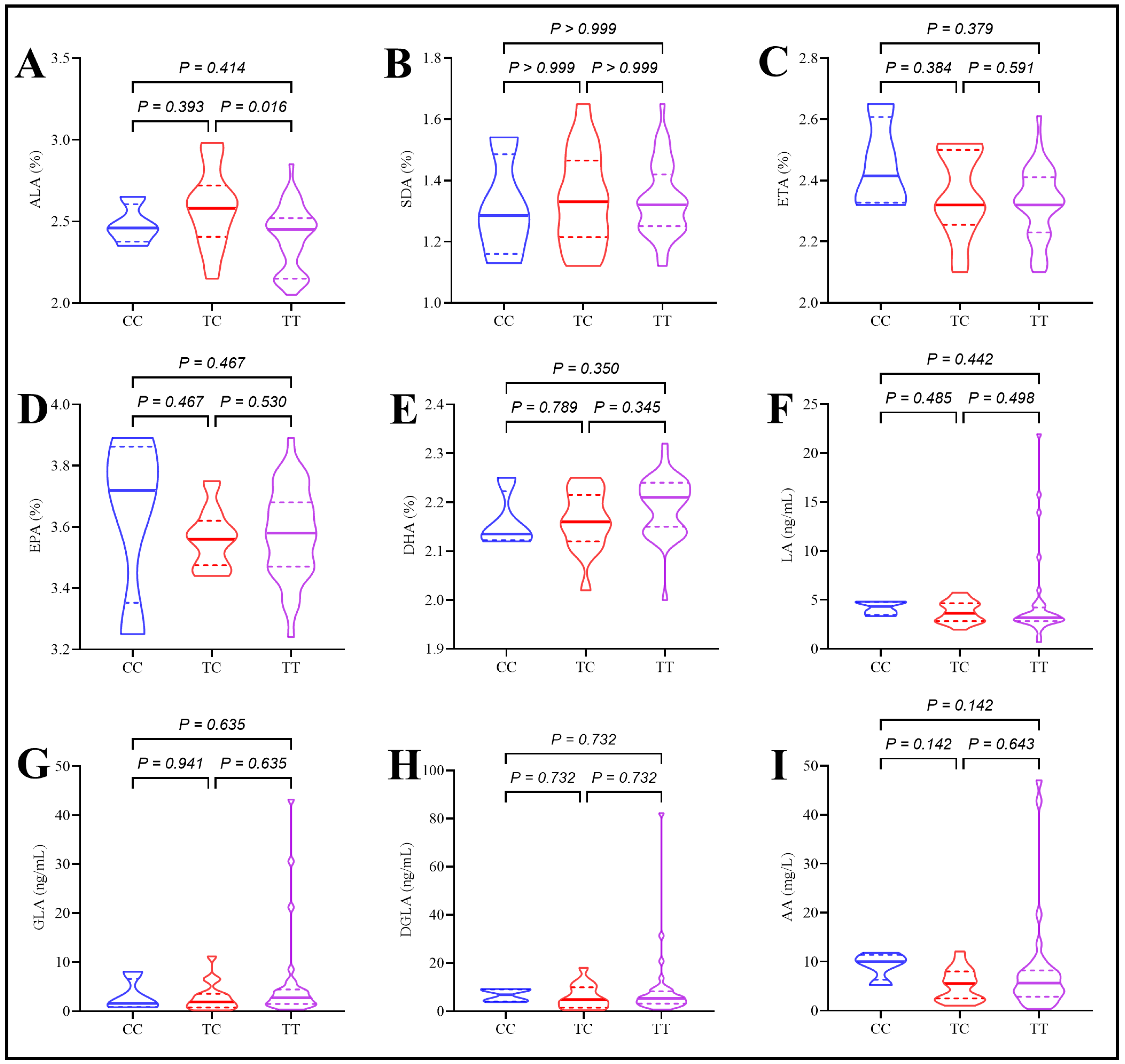

| Variables | Control | T2DM | p-Value |
|---|---|---|---|
| Number | 60 | 60 | - |
| Age (years) a | 45.6 ± 6.7 | 47.8 ± 9.9 | 0.160 d |
| Sex b | 0.264 e | ||
| Female | 27 (45.0%) | 21 (35.0%) | |
| Male | 33 (55.0%) | 39 (65.0%) | |
| BMI (kg/m2) a | 27.2 ± 2.1 | 27.0 ± 2.0 | 0.566 d |
| WHR a | 0.837 ± 0.041 | 0.841 ± 0.039 | 0.552 d |
| Education levels b | 0.484 e | ||
| Illiterate | 6 (10.0%) | 11 (18.3%) | |
| Primary or secondary | 51 (85.0%) | 47 (78.3%) | |
| College | 3 (5.0%) | 2 (3.3%) | |
| Fish diet b | 0.575 e | ||
| Once every two weeks | 10 (16.7%) | 14 (23.3%) | |
| Once weekly | 33 (55.0%) | 28 (46.7%) | |
| Twice weekly | 17 (28.3%) | 18 (30.0%) | |
| FPG (mg/dL) c | 98 (93–105) | 136.5 (119.3–198.8) | <0.001 f |
| HbA1c (%) a | 5.73 ± 0.41 | 9.50 ± 2.42 | <0.001 d |
| Fasting insulin (µU/mL) c | 8.6 (4.52–11.85) | 10.21 (5.89–18.96) | 0.009 f |
| HOMA-IR c | 2.2 (1.11–2.78) | 3.63 (2.34–7.04) | <0.001 f |
| Apo A (g/L) a | 1.28 ± 0.25 | 1.21 ± 0.36 | 0.210 d |
| Apo B (g/L) a | 1.15 ± 0.22 | 1.15 ± 0.25 | 0.920 d |
| Apo B/A ratio c | 0.87 (0.75–1.11) | 0.90 (0.82–1.16) | 0.320 f |
| Cholesterol (mg/DL) a | 173.8 ± 31.7 | 182.1 ± 45.6 | 0.250 d |
| LDL-c (mg/dL) a | 110.6 ± 24.9 | 111.7 ± 39.3 | 0.860 d |
| HDL-c (mg/dL) a | 39.95 ± 6.91 | 35.72 ± 7.66 | 0.002 d |
| Triglyceride (mg/dL) c | 108.5 (88.5–142.8) | 167.5 (118.8–215.0) | <0.001 f |
| VLDL (mg/dL) c | 21.9 (17.7–21.9) | 32.9 (23.7–42.95) | <0.001 f |
| hs-CRP (mg/L) c | 1.78 (0.6–4.27) | 3.28 (1.16–5.83) | 0.020 f |
| ω3-PUFA | |||
| ALA (%) a | 4.014 ± 0.413 | 2.46 ± 0.217 | <0.001 d |
| SDA (%) a | 2.482 ± 0.216 | 1.334 ± 0.131 | <0.001 d |
| ETA (%) a | 3.353 ± 0.160 | 2.34 ± 0.126 | <0.001 d |
| EPA (%) a | 4.378 ± 0.172 | 3.581 ± 0.143 | <0.001 d |
| DHA (%) a | 2.513 ± 0.102 | 2.182 ± 0.061 | <0.001 d |
| ω6-PUFA | |||
| LA (ng/mL) c | 4.71 (3.19–7.09) | 3.41 (2.86–4.49) | 0.009 f |
| GLA (ng/mL) c | 4.32 (2.98–5.69) | 2.44 (1.24–4.21) | 0.001 f |
| DGLA (ng/mL) c | 12.13 (6.94–19.34) | 4.91 (3.24–8.79) | <0.001 f |
| AA (mg/L) | 10.41 (5.997–14.71) | 5.65 (2.57–8.34) | <0.001 f |
| Models | Genotype | Control | T2DM | OR (95% CI) | p-Value |
|---|---|---|---|---|---|
| Co-dominant | CC | 2 (3.3%) | 4 (6.7%) | 2.615 (0.455–15.018) | 0.281 a |
| TC | 7 (11.7%) | 17 (28.3%) | 3.176 (1.199–8.411) | 0.020 a | |
| TT | 51 (85.0%) | 39 (65.0%) | 1.0 | - | |
| Dominant | CC + TC | 9 (15.0%) | 21 (35.0%) | 3.051 (1.259–7.395) | 0.014 a |
| TT | 51 (85.0%) | 39 (65.0%) | 1.0 | - | |
| Recessive | TT + TC | 58 (96.7%) | 56 (93.3%) | 0.483 (0.085–2.741) | 0.411 a |
| CC | 2 (3.3%) | 4 (6.7%) | 1.0 | - | |
| Allele | C | 11 (9.17%) | 25 (20.83%) | - | 0.011 b |
| T | 109 (90.83%) | 95 (79.17%) | - |
| Models | OR (95% CI) | p-Value |
|---|---|---|
| Unadjusted a | 3.051 (1.259–7.395) | 0.014 |
| Model-1 b | 3.266 (1.279–8.338) | 0.013 |
| Model-2 c | 3.230 (1.034–10.087) | 0.044 |
| Model-3 d | 3.967 (1.167–13.480) | 0.027 |
| Model-4 e | 3.070 (0.777–12.135) | 0.110 |
| Variables a | CC | TC | TT | p-Value b |
|---|---|---|---|---|
| Number | 4 | 17 | 39 | - |
| D5D (pg/mL) | 164.5 (118.6–262.5) | 177.7 (113.6–307.3) | 134.9 (85.1–213.8) | 0.243 |
| EPA/ETA ratio (D5D) | 1.5 (1.3–1.7) | 1.5 (1.5–1.6) | 1.5 (1.5–1.6) | 0.480 |
| AA/DGLA ratio (D5D) | 1625.7 (768.4–2561.5) | 825.3 (494.7–2533.5) | 1075.9 (688.7–1809.6) | 0.701 |
| D6D (ng/mL) | 79.9 (58.9–97.8) | 89.6 (55.6–104.7) | 76.7 (60.4–102.2) | 0.986 |
| SDA/ALA ratio (D6D) | 0.5 (0.5–0.6) | 0.5 (0.5–0.6) | 0.6 (0.5–0.6) | 0.188 |
| GLA/LA ratio (D6D) | 0.5 (0.2–1.4) | 0.6 (0.2–1.3) | 0.8 (0.5–1.3) | 0.500 |
| ETA/SDA ratio (elongase-5) | 1.8 (1.7–2.1) | 1.7 (1.6–1.9) | 1.8 (1.6–1.9) | 0.562 |
| DGLA/GLA ratio (elongase-5) | 3.3 (1.3–9.5) | 1.7 (1.1–3.1) | 2.1 (1.1–3.0) | 0.692 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jarullah, H.H.; Saleh, E.S. Influence of Fatty Acid Desaturase Enzyme-1 Gene (FADS-1) Polymorphism on Serum Polyunsaturated Fatty Acids Levels, Desaturase Enzymes, Lipid Profile, and Glycemic Control Parameters in Newly Diagnosed Diabetic Mellitus Patients. Int. J. Mol. Sci. 2025, 26, 4015. https://doi.org/10.3390/ijms26094015
Jarullah HH, Saleh ES. Influence of Fatty Acid Desaturase Enzyme-1 Gene (FADS-1) Polymorphism on Serum Polyunsaturated Fatty Acids Levels, Desaturase Enzymes, Lipid Profile, and Glycemic Control Parameters in Newly Diagnosed Diabetic Mellitus Patients. International Journal of Molecular Sciences. 2025; 26(9):4015. https://doi.org/10.3390/ijms26094015
Chicago/Turabian StyleJarullah, Hayder Huwais, and Eman Saadi Saleh. 2025. "Influence of Fatty Acid Desaturase Enzyme-1 Gene (FADS-1) Polymorphism on Serum Polyunsaturated Fatty Acids Levels, Desaturase Enzymes, Lipid Profile, and Glycemic Control Parameters in Newly Diagnosed Diabetic Mellitus Patients" International Journal of Molecular Sciences 26, no. 9: 4015. https://doi.org/10.3390/ijms26094015
APA StyleJarullah, H. H., & Saleh, E. S. (2025). Influence of Fatty Acid Desaturase Enzyme-1 Gene (FADS-1) Polymorphism on Serum Polyunsaturated Fatty Acids Levels, Desaturase Enzymes, Lipid Profile, and Glycemic Control Parameters in Newly Diagnosed Diabetic Mellitus Patients. International Journal of Molecular Sciences, 26(9), 4015. https://doi.org/10.3390/ijms26094015






