Rotation of Hexamethylenetetramine Molecules Induces Reversible Electromagnetic Coupling Properties in Isothiocyanato-Nickel Complexes
Abstract
1. Introduction
2. Results and Discussion
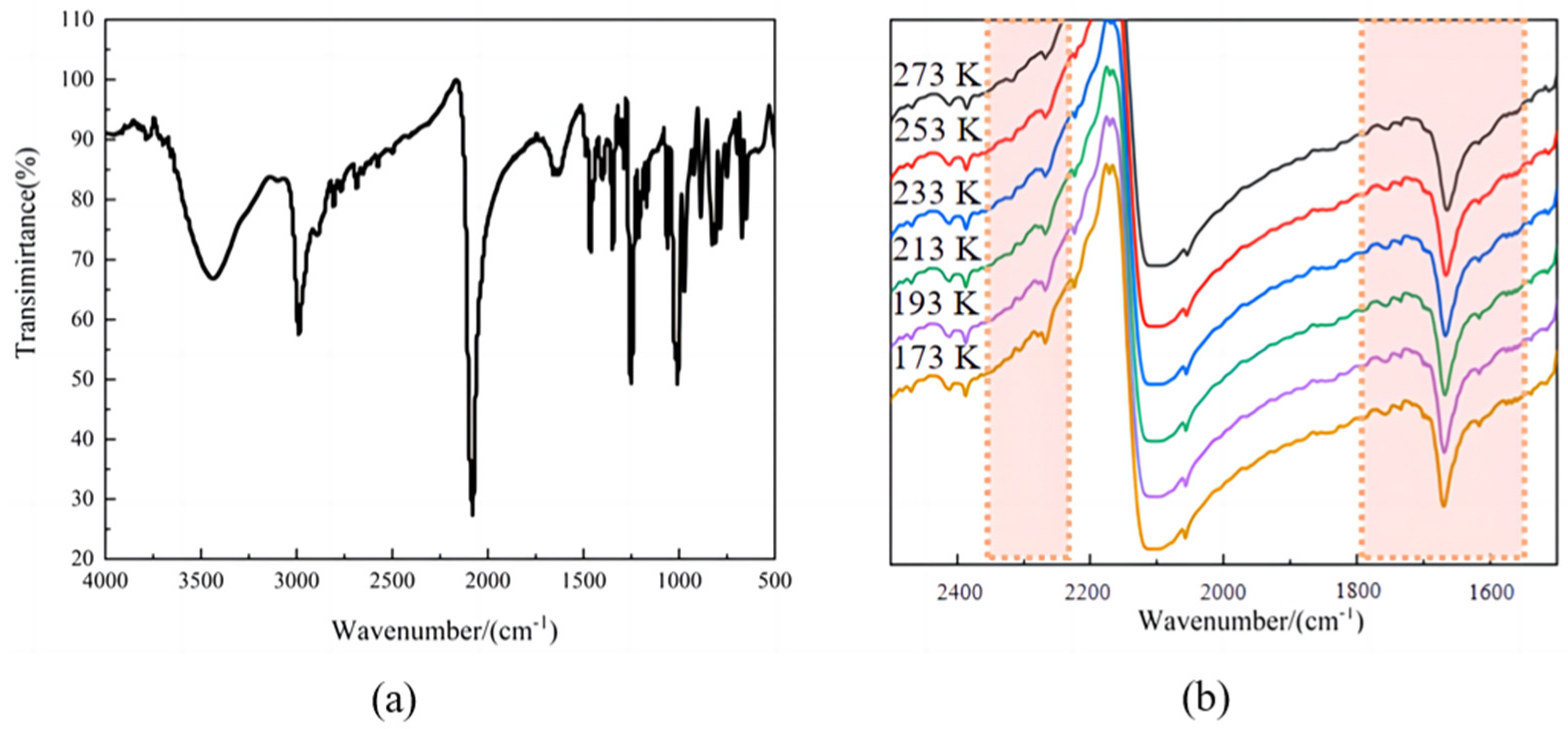
2.1. Variable-Temperature IR Spectra of Compound 1
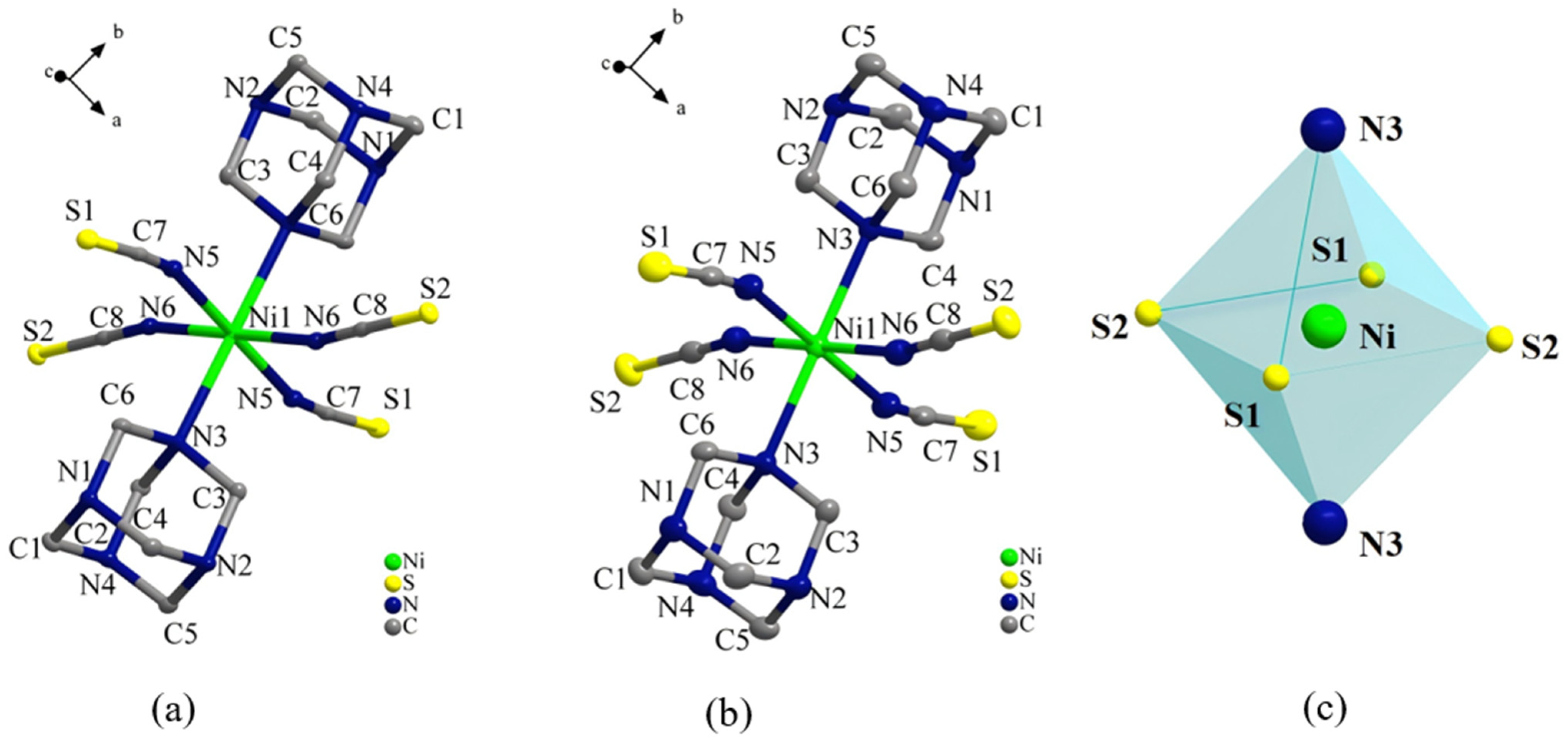
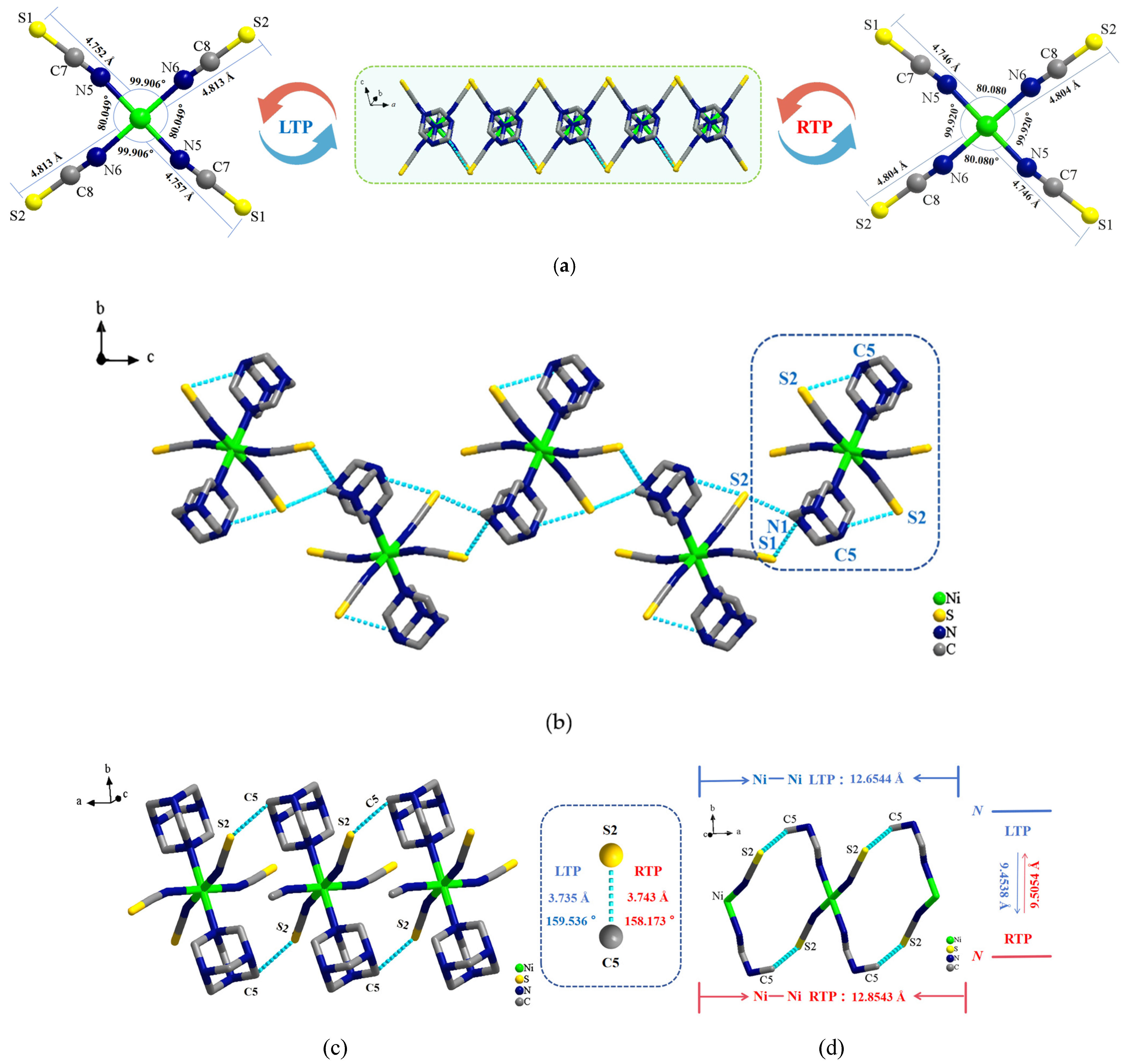
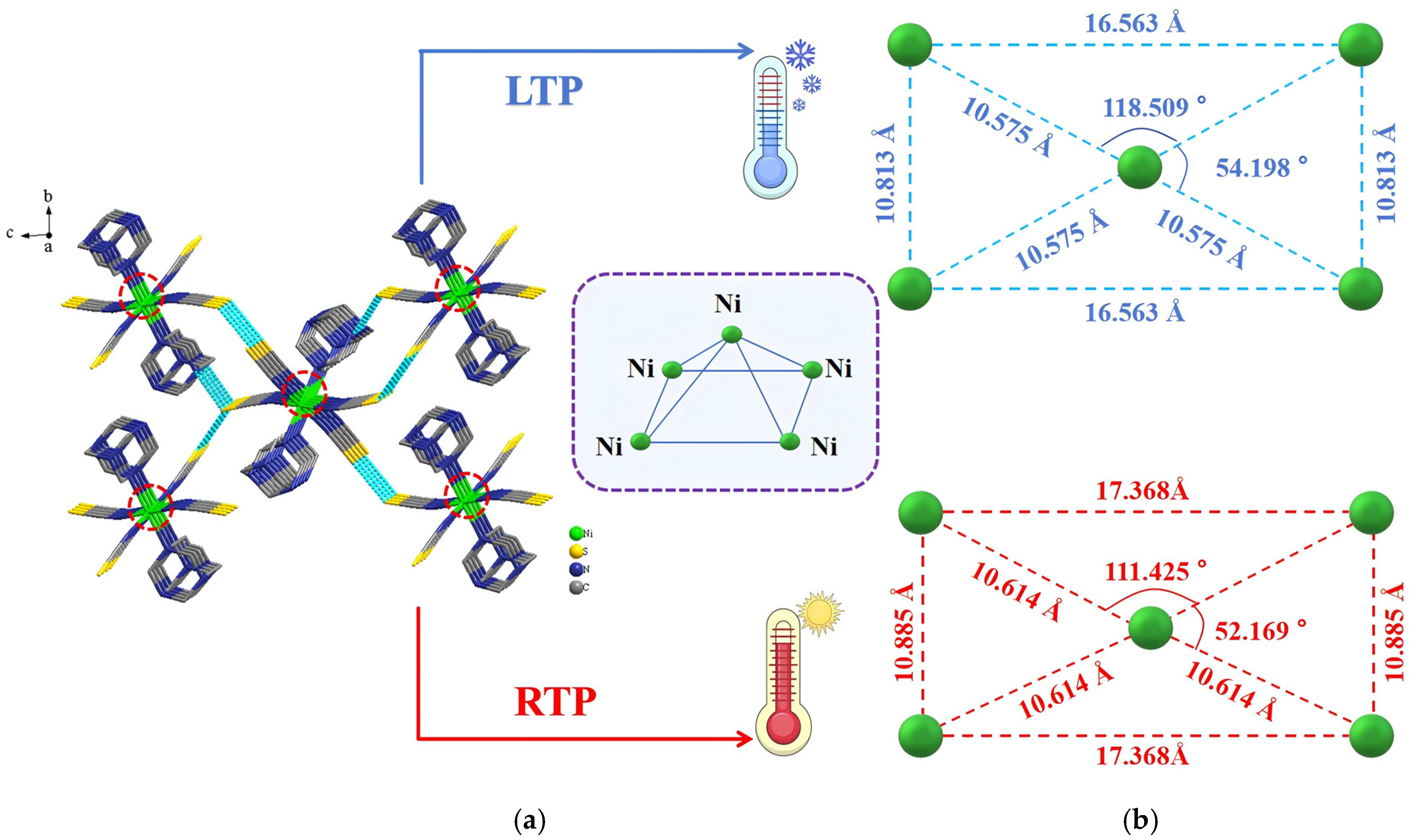
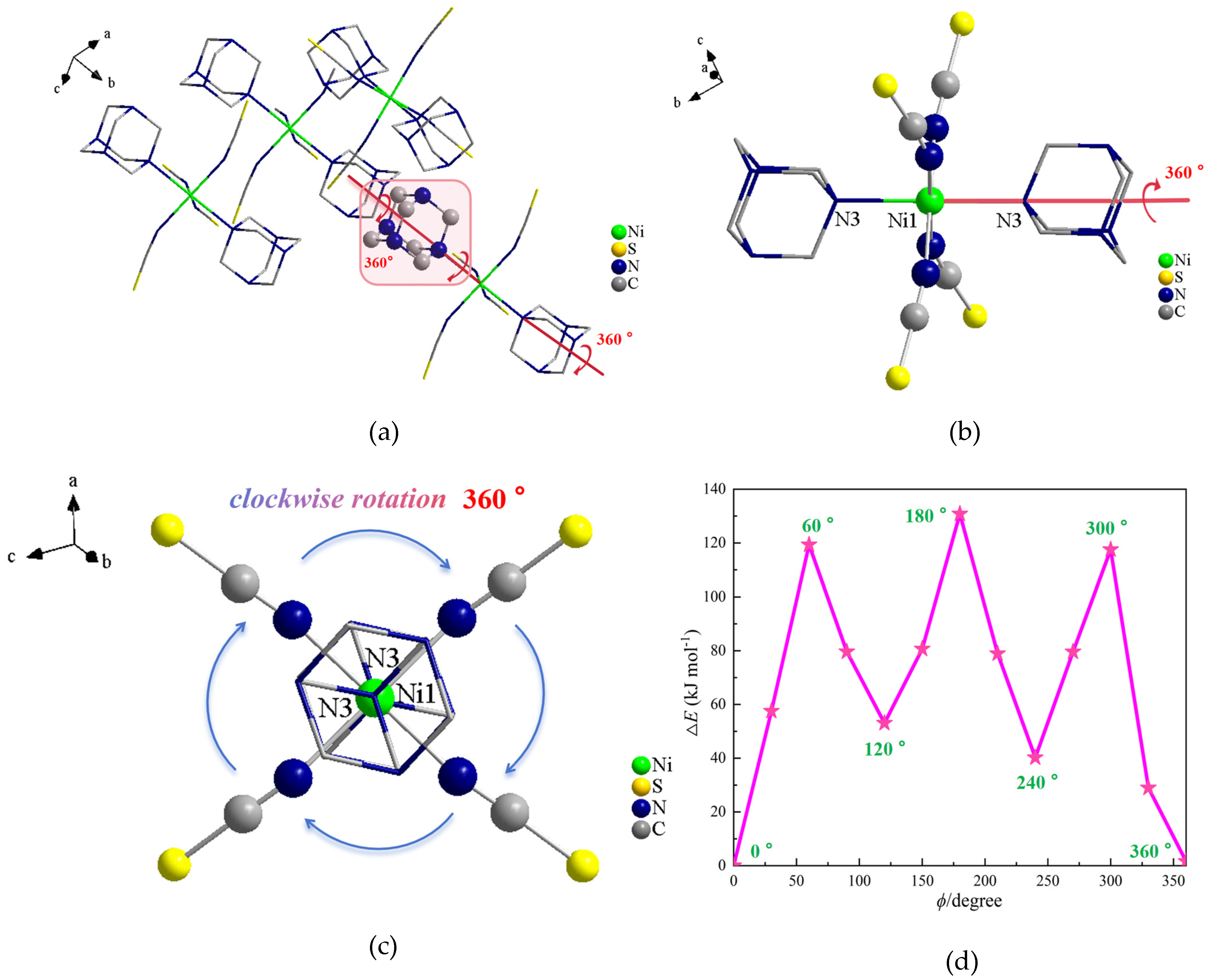
2.2. Single-Crystal X-Ray Structures of Compound 1
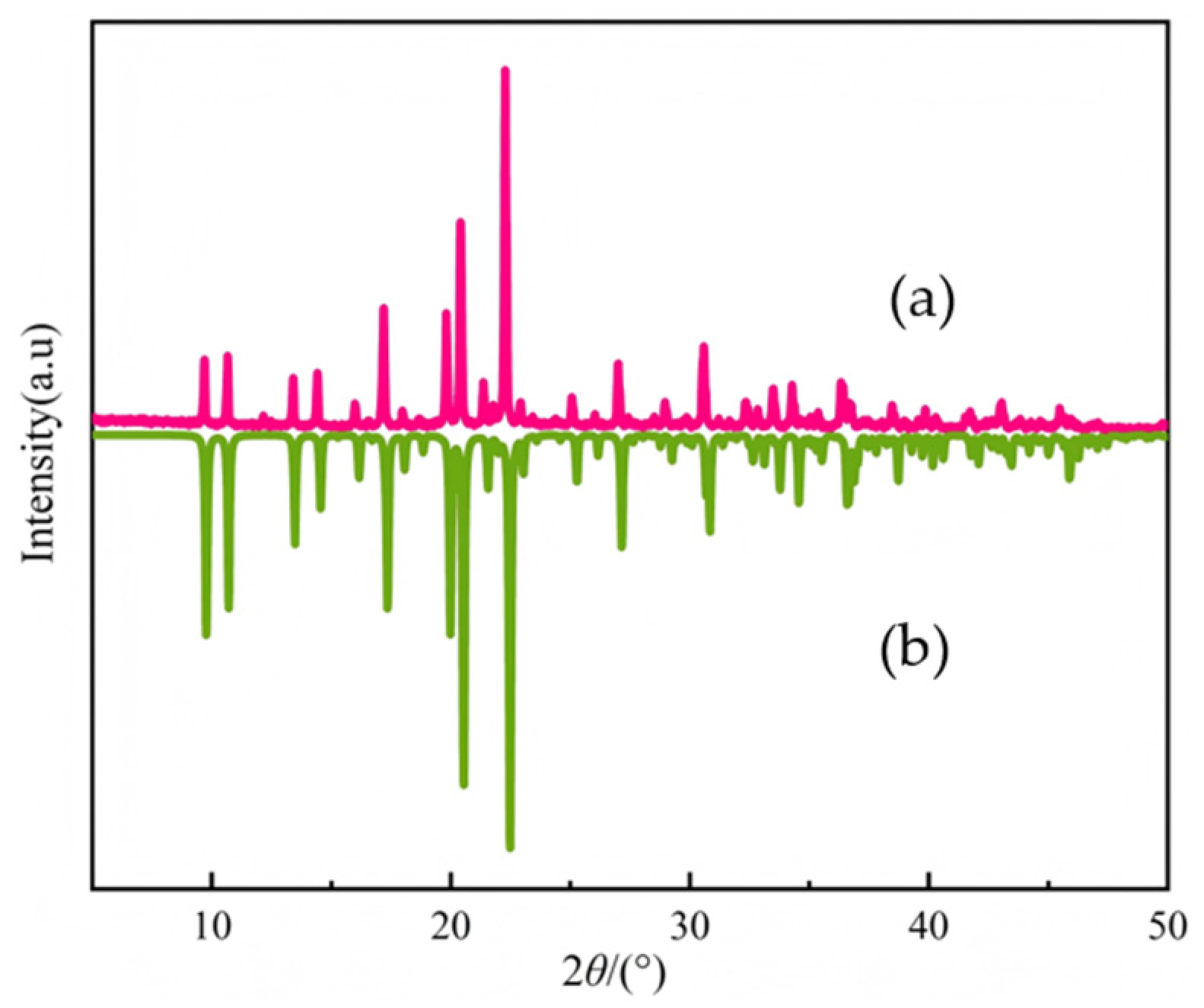
2.3. XRD Spectrum of Compound 1
2.4. Thermal Analysis of Compound 1
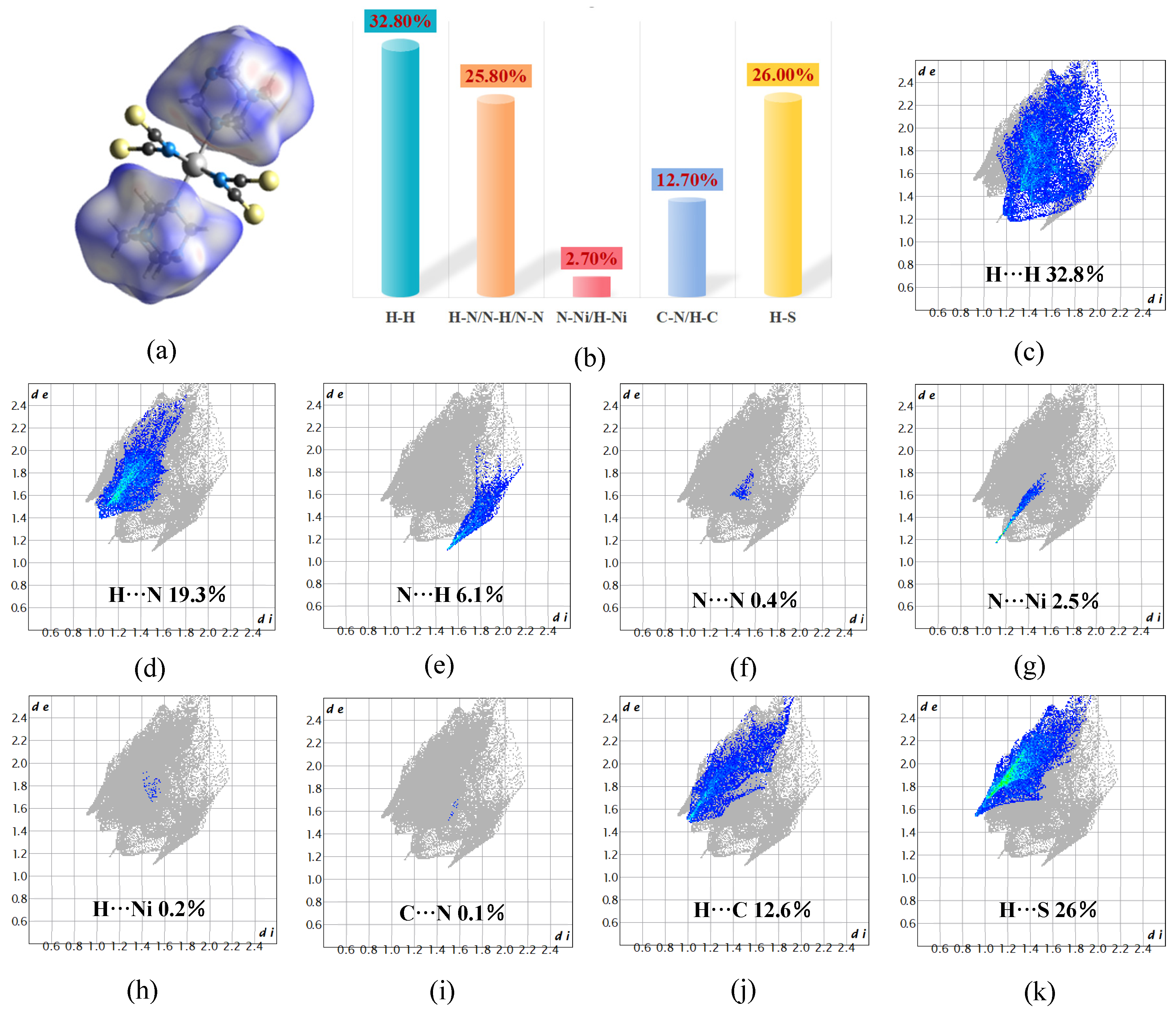
2.5. Hirshfeld Surface Analysis of Compound 1
2.6. Potential Energy of Cation Rotation in Compound 1
2.7. Semiconducting Properties of Compound 1
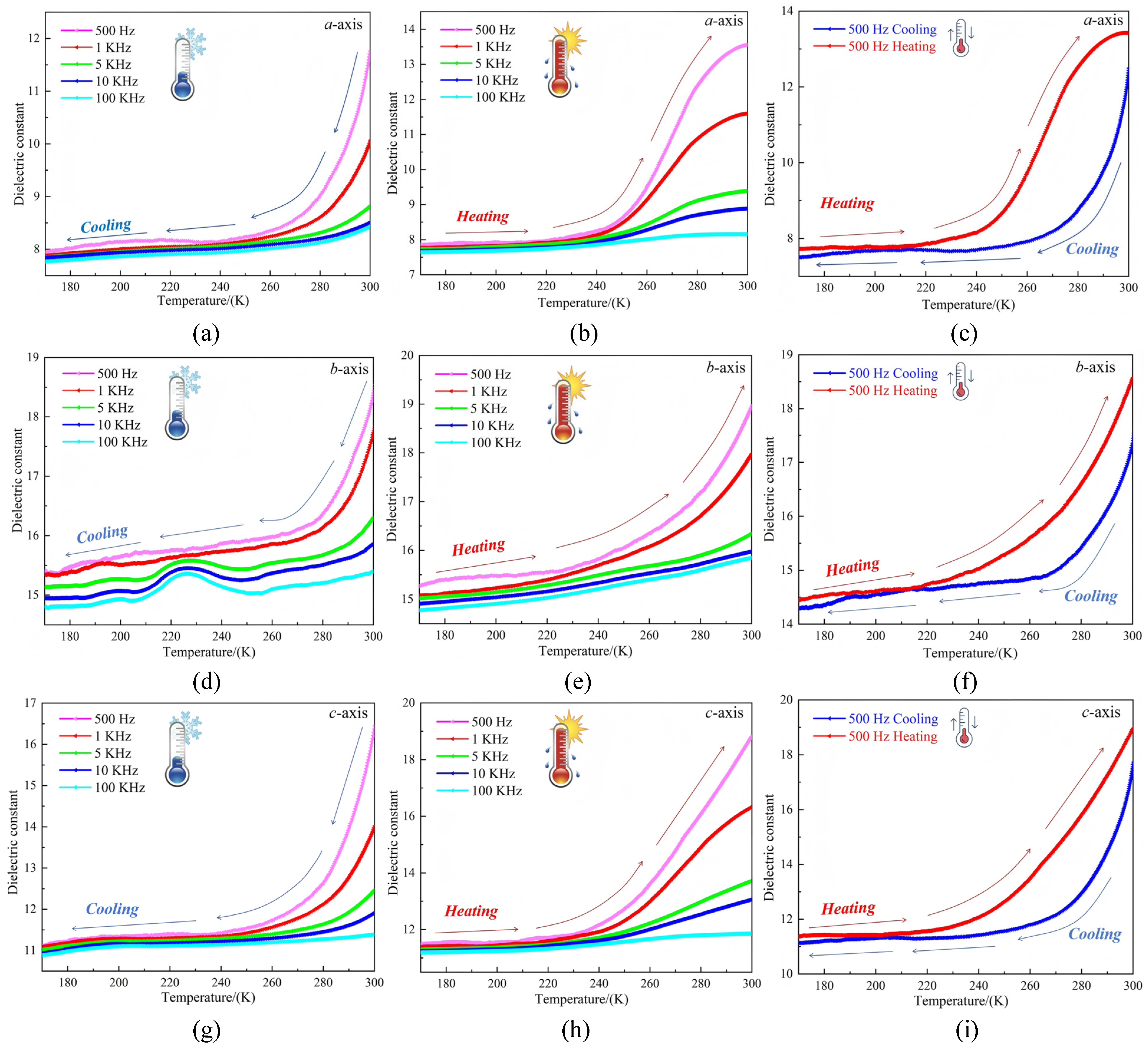
2.8. Dielectric Properties of Compound 1
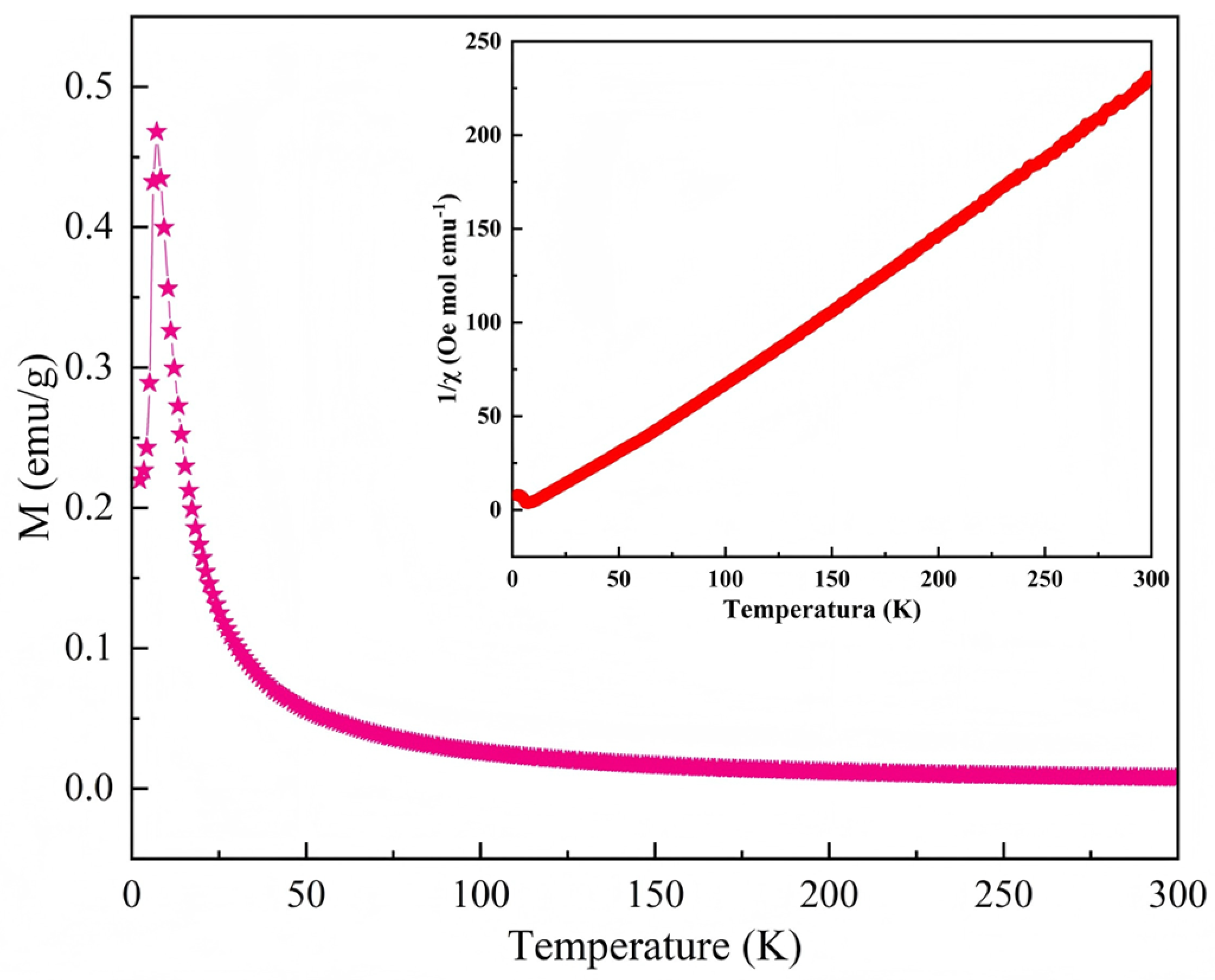
2.9. Magnetic Testing of Compound 1
2.10. Cyclic Voltammetry (CV) of Compound 1
3. Materials and Methods
3.1. Main Reagents and Instruments
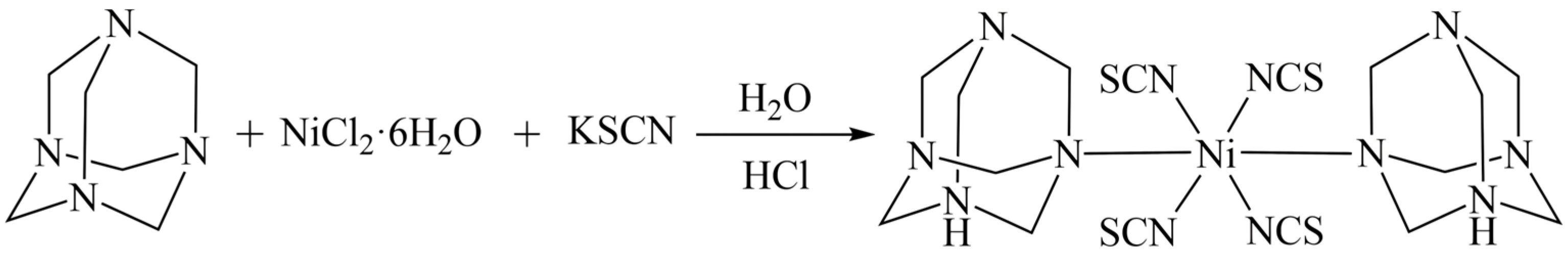
3.2. Synthesis Methods
3.3. Measurement of Crystal Structure of Compound 1
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, D.; Li, J.; Mei, X.; Liu, X.; Zuo, P.; Qiu, X.; Zhuang, Q. Adjustable Plane Curvature of Covalent Organic Framework Enabling Outstanding Dielectric, Electret, and High-Temperature Processing Properties. Angew. Chem. 2023, 62, e202315143. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xu, Z.K.; Wang, Z.X.; Yu, H.; Lv, H.P.; Li, P.F.; Liao, W.Q.; Xiong, R.G. Large Piezoelectric Response in a Metal-Free Three-Dimensional Perovskite Ferroelectric. J. Am. Chem. Soc. 2023, 145, 4892–4899. [Google Scholar] [CrossRef] [PubMed]
- Wu, M. Two-Dimensional van der Waals Ferroelectrics: Scientific and Technological Opportunities. ACS Nano 2021, 15, 9229–9237. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, A.; Acharya, M.; Lee, H.G.; Schimpf, J.; Jiang, Y.; Lou, D.; Tian, Z.; Martin, L.W. Thin-Film Ferroelectrics. Adv. Mater. 2022, 34, 2108841. [Google Scholar] [CrossRef]
- Cui, Z.; Cao, S.; Huang, C.; Chen, Y.; Zhang, M.; Wang, A.; Xiao, X. Broad Detection Range of Flexible Capacitive Sensor with 3D Printed Interwoven Hollow Dual-Structured Dielectric Layer. Appl. Mater. Today 2024, 36, 102064. [Google Scholar] [CrossRef]
- Charytanowicz, T.; Wang, J.; Tokoro, H.; Tran, K.; Renz, F.; Ohkoshi, S.I.; Chorazy, S.; Sieklucka, B. Thermal Bistability of Magnetic Susceptibility, Light Absorption, Second Harmonic Generation, and Dielectric Properties in a Polar Spin-Crossover Iron–Rhenium Chain Material. Angew. Chem. 2024, 136, e202419242. [Google Scholar]
- Huang, X.Y.; Luo, Y.L.; Zhu, X.; Deng, X.; Yan, X.; Wang, Y.J.; Zhou, L.; Tang, Y.Y. A Sn-Based Hybrid Ferroelastic Semiconductor with High-Temperature Dielectric Switching. Inorg. Chem. 2024, 63, 2525–2532. [Google Scholar] [CrossRef]
- Senarathna, M.C.; Li, H.; Perera, S.D.; Torres-Correas, J.; Diwakara, S.D.; Boardman, S.R.; Al-Kharji, N.M.; Liu, Y.; Smaldone, R.A. Highly Flexible Dielectric Films from Solution Processable Covalent Organic Frameworks. Angew. Chem. Int. Ed. 2023, 62, e202312617. [Google Scholar] [CrossRef]
- Ying, T.T.; Shi, H.J.; Chen, S.P.; Tang, Y.Z.; Tan, Y.H.; Wang, S.F.; Sun, Z.; Wang, F.X.; Wan, M.Y. Large Spontaneous Polarization Ferroelectric Property, Switchable Second-Harmonic Generation Responses, and Magnetism in an Fe-Based Compound. Inorg. Chem. 2023, 62, 6189–6195. [Google Scholar] [CrossRef]
- Deng, B.W.; Zhu, Y.; Ding, K.; Li, J.; Lun, M.M.; Fu, D.W.; Zhang, Z.X. Homochiral Chemistry Strategy to Trigger Second-Harmonic Generation and Dual Dielectric Switches. Inorg. Chem. 2023, 62, 11701–11707. [Google Scholar] [CrossRef]
- Ning, Y.; Yang, D.B.; Yang, S.; Zhang, Y.; Saven, J.G.; Murray, C.B. Aligning the Induced Anisotropy of Isotropic Nanoparticles with Liquid Crystals. J. Am. Chem. Soc. 2025, 147, 16332. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Qi, J.C.; Liao, W.Q. Optically Controlled Polarization Switching in an Organic Ferroelectric with Light- and Temperature-Triggered Phase Transitions. Chem. Mater. 2022, 34, 3067–3075. [Google Scholar] [CrossRef]
- Hooijer, R.; Wang, S.; Biewald, A.; Eckel, C.; Righetto, M.; Chen, M.; Xu, Z.; Blätte, D.; Han, D.; Ebert, H.; et al. Overcoming Intrinsic Quantum Confinement and Ultrafast Self-Trapping in Ag–Bi–I- and Cu–Bi–I-Based 2D Double Perovskites through Electroactive Cations. J. Am. Chem. Soc. 2024, 146, 26694–26706. [Google Scholar] [CrossRef]
- Teri, G.; Ni, H.F.; Luo, Q.F.; Wang, X.P.; Wang, J.Q.; Fu, D.W.; Guo, Q. Tin-Based Organic–Inorganic Metal Halides with a Reversible Phase Transition and Thermochromic Response. Mater. Chem. Front. 2023, 7, 2235–2240. [Google Scholar] [CrossRef]
- Gong, J.M.; Shao, T.; Huang, P.Z.; Su, C.Y.; Chen, M.; Fu, D.W.; Lu, H.F. Reversible Phase Transition and Second-Harmonic Response Based on a Zero-Dimensional Organic-Inorganic Hybrid Compound. J. Phys. Chem. C 2022, 126, 15274–15279. [Google Scholar] [CrossRef]
- Xu, K.; Zhou, Z.N.; Han, X.B.; Yang, Y.W.; Zhang, W.; Ye, Q. Shape Shifting and Locking in Mechanically Responsive Organic-Inorganic Hybrid Materials for Thermoelastic Actuators. Angew. Chem. 2024, 136, e202408247. [Google Scholar] [CrossRef]
- Balashova, E.; Levin, A.A.; Fokin, A.; Redkov, A.; Krichevtsov, B. Structural Properties and Dielectric Hysteresis of Molecular Organic Ferroelectric Grown from Different Solvents. Crystals 2021, 11, 1278. [Google Scholar] [CrossRef]
- Li, M.; Xu, Y.; Han, S.; Xu, J.; Xie, Z.; Liu, Y.; Xu, Z.; Hong, M.; Luo, J.; Sun, Z. Giant and Broadband Multiphoton Absorption Nonlinearities of a 2D Organometallic Perovskite Ferroelectric. Adv. Mater. 2020, 32, 2002972. [Google Scholar] [CrossRef]
- Li, Z.L.; Ni, H.F.; Zhuang, B.; Ding, K.; Fu, D.W.; Guo, Q.; Lun, M.M. Halogen Substitution Strategy for Regulating the Photoluminescence and Dielectric Response of Ferroelastics. Chin. Chem. Lett. 2024, 110755. [Google Scholar] [CrossRef]
- Yao, N.T.; Shang, M.J.; Lu, H.H.; Liu, Q.; Wen, W.; Zhang, S.H.; Zhao, L.; Oshio, H.; Meng, Y.S.; Liu, T. Hysteretic Magnetism–Dielectricity Switching in 2D Hofmann Type Spin-Crossover Compounds. Inorg. Chem. Front. 2024, 11, 3159–3167. [Google Scholar] [CrossRef]
- Song, J.; Gong, M.; Tsai, M.F.; Ma, Y.; Ma, H.; Liu, Y.; Chu, Y.H.; Huang, R.; Ouyang, J.; Wang, J.; et al. Pseudo-Ferroelectric Domain-Wall in Perovskite Ferroelectric Thin Films. Adv. Funct. Mater. 2023, 33, 2300330. [Google Scholar] [CrossRef]
- Zhu, M.; Huang, P.Z.; Li, L.M.; Yang, Y.X.; Pan, L.; Wang, Z.J.; Ni, H.F.; Zhang, F.W.; Teri, G.; Zhang, Z.X.; et al. Thermal-Responsive Luminescence/Dielectric Responses with Reversibly Shifted Light Emissions. Chem. Sci. 2025, 16, 4101–4108. [Google Scholar] [CrossRef]
- Wang, K.; Guo, X.; Han, C.; Liu, L.; Wang, Z.; Thomsen, L.; Chen, P.; Shao, Z.; Wang, X.; Xie, F.; et al. Creation of Piezoelectricity in Quadruple Perovskite Oxides by Harnessing Cation Defects and Their Application in Piezo-Photocatalysis. ACS Nano 2025, 19, 3818–3829. [Google Scholar] [CrossRef] [PubMed]
- Ai, Y.; Liao, W.Q.; Weng, Y.R.; Lv, H.P.; Chen, X.G.; Song, X.J.; Li, P.F.; Xiong, R.G. Discovery of Ferroelectricity in the Fullerene Adduct C60S8. J. Am. Chem. Soc. 2023, 145, 23292–23299. [Google Scholar] [CrossRef]
- Xiao, L.; Xu, X.; Lu, Z.; Zhao, J.; Liu, R.; Ye, Y.; Tang, R.; Liao, W.Q.; Xiong, R.G.; Zou, G. In-Situ Organic-Inorganic Ferroelectric Layer Growth for Efficient Perovskite Solar Cells with High Photovoltage. Nano Energy 2023, 107, 108114. [Google Scholar] [CrossRef]
- Zhou, Z.; Yang, Y.; Cao, P.; Zhang, S.; Ye, Q. Spontaneous Polarization Halide Perovskite Piezo-Photocatalysis for Highly Efficient Photocatalytic Hydrogen Evolution. J. Colloid Interface Sci. 2025, 685, 1018–1026. [Google Scholar] [CrossRef] [PubMed]
- Dey, P.; Islam, S.; Seth, S.K. Quantitative Analysis of the Interplay of Hydrogen Bonds in M (II)-Hexaaqua Complexes with HMTA [M (II) = Co (II), Mg (II); HMTA = Hexamethylenetetramine]. J. Mol. Struct. 2023, 1284, 135448. [Google Scholar] [CrossRef]
- Milo, T.N.; Tabong, C.D.; Nono, J.H.; Bekindaka, E.D.; Yufanyi, D.M.; Agwara, M.O. Hydrated Magnesium (II) with H-Bonded Hexamethylenetetramine and Co-Ligands: Synthesis, Structural Characterization, Thermal Decomposition, and Hirshfeld Surface Analysis. Chem. Pap. 2022, 76, 6385–6400. [Google Scholar] [CrossRef]
- Gong, Y.Y.; Zhang, T.; Li, J.; Fu, D.W.; Zhang, Y.; Lu, H.F. Structural Optimization and Property Tunability by Halogen Regulation in Zero-Dimensional Zinc Halide Organic–Inorganic Hybrid Materials. Cryst. Growth Des. 2022, 22, 6801–6808. [Google Scholar] [CrossRef]
- McArthur, J.; Filip, M.R.; Qiu, D.Y. Minimal Molecular Building Blocks for Screening in Quasi-Two-Dimensional Organic–Inorganic Lead Halide Perovskites. Nano Lett. 2023, 23, 3796–3802. [Google Scholar] [CrossRef]
- Chen, Q.Q.; Hu, C.L.; Chen, J.; Li, Y.L.; Li, B.X.; Mao, J.G. [o-C5H4NHOH]2[I7O18(OH)]·3H2O: An Organic-Inorganic Hybrid SHG Material Featuring an [I7O18(OH)] Branched Polyiodate Chain. Angew. Chem. 2021, 133, 17566–17569. [Google Scholar] [CrossRef]
- Sugiura, Y.; Makita, Y. Intercalated Molecule Releasing Process of Thiomalate Substituted Octacalcium Phosphate Crystals During Phase Conversion. J. Cryst. Growth 2022, 583, 126545. [Google Scholar] [CrossRef]
- Mizoue, R.; Takeda, T.; Dekura, S.; Kato, M.; Fukui, T.; Shoji, Y.; Fukushima, T.; Yamane, S.; Suzuki, Y.; Kawamata, J.; et al. Ferroelectricity of Alkylamide-Substituted Triptycene Derivatives. J. Mater. Chem. C 2024, 12, 5578–5586. [Google Scholar] [CrossRef]
- Fu, D.W.; Gao, J.X.; Huang, P.Z.; Ren, R.Y.; Shao, T.; Han, L.J.; Liu, J.; Gong, J.M. Observation of Transition from Ferroelasticity to Ferroelectricity by Solvent Selective Effect in Anilinium Bromide. Angew. Chem. Int. Ed. 2021, 60, 8198–8202. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Takahashi, K.; Huang, R.K.; Xue, C.; Kokado, K.; Hoshino, N.; Akutagawa, T.; Nishihara, S.; Nakamura, T. Multifunctional Triggering by Solid-Phase Molecular Motion: Relaxor Ferroelectricity, Modulation of Magnetic Exchange Interactions, and Enhancement of Negative Thermal Expansion. Chem. Mater. 2023, 35, 2421–2428. [Google Scholar] [CrossRef]
- Ho, M.R.; Wu, Y.M.; Lu, Y.C.; Ko, T.P.; Wu, K.P. Cryo-EM Reveals the Structure and Dynamics of a 723-Residue Malate Synthase G. J. Struct. Biol. 2023, 215, 107958. [Google Scholar] [CrossRef]
- Gong, Y.; Zhao, J.; Li, Z.; Huang, J.; Zhang, Y.; Dong, L.; Xiong, C.; Jiang, M. Unparalleled Dielectric-Switching Effects Caused by Dual Polarization Synergy. Adv. Funct. Mater. 2023, 33, 2214544. [Google Scholar] [CrossRef]
- Kotani, Y.; Katsuno, R.; Sambe, K.; Terasaki, S.; Matsuno, T.; Ikemoto, K.; Akutagawa, T.; Isobe, H. Manipulating the Dynamics of Chiral Molecular Bearings Through Subtle Structural Changes of Guest Molecules. Angew. Chem. Int. Ed. 2024, 64, e202425308. [Google Scholar] [CrossRef]
- Zhang, T.; Xu, K.; Li, J.; He, L.; Fu, D.W.; Ye, Q.; Xiong, R.G. Ferroelectric Hybrid Organic–Inorganic Perovskites and Their Structural and Functional Diversity. Natl. Sci. Rev. 2023, 10, nwac240. [Google Scholar] [CrossRef]
- Peng, Q.; Li, D.; Huang, P.; Ren, Y.; Li, Z.; Pi, L.; Chen, P.; Wu, M.; Zhang, X.; Zhou, X.; et al. Room-Temperature Ferroelectricity in 2D Metal-Tellurium–Oxyhalide Cd7Te7Cl8O17 via Selenium-Induced Selective-Bonding Growth. ACS Nano 2021, 15, 16525–16532. [Google Scholar] [CrossRef]
- Sato, C.; Dekura, S.; Sato, H.; Sambe, K.; Takeda, T.; Kurihara, T.; Mizuno, M.; Taniguchi, T.; Wu, J.; Nakamura, T.; et al. Proton Conduction in Chiral Molecular Assemblies of Azolium–Camphorsulfonate Salts. J. Am. Chem. Soc. 2024, 146, 22699–22710. [Google Scholar] [CrossRef]
- Sarkar, S.; Ghosh, S.R.; Brandão, P.; Jana, A.D. Role of Imidazole Edge to Edge Supramolecular Interaction in the Crystal Packing of Cu(II)(SCN−)2(Imidazole)2 Complex: A Novel Variety of Supramolecular Interaction Revealed by CCDC Database Analysis and Explored through DFT Computational Studies. J. Mol. Struct. 2021, 1227, 129513. [Google Scholar] [CrossRef]
- Wang, F.; Ju, L.; Wu, B.; Li, S.; Peng, J.; Chen, Y.; Getaye Sendeku, M.; Wang, K.; Cai, Y.; Yi, J.; et al. Effect of Intrinsic Ferroelectric Phase Transition on Hydrogen Evolution Electrocatalysis. Angew. Chem. 2024, 136, e202402033. [Google Scholar] [CrossRef]
- Feng, D.; Zhu, Z.; Chen, X.; Qi, J. Electric-Polarization-Driven Magnetic Phase Transition in a Ferroelectric–Ferromagnetic Heterostructure. Appl. Phys. Lett. 2021, 118, 060601. [Google Scholar] [CrossRef]
- Szeremeta, A.Z.; Macutkevič, J.; Zubko, M.; Miga, S.; Svirskas, Š.; Gruszka, I.; Koperski, J.; Banys, J.; Molak, A. Doping Influence on Structural Ferroelectric Phase Transitions and Electrical Features of Barium Calcium Titanate. J. Eur. Ceram. Soc. 2023, 43, 4029–4043. [Google Scholar] [CrossRef]
- Wang, Z.J.; Shen, M.J.; Rao, Z.P.; Huang, P.Z.; Lun, M.M.; Deng, B.W.; Li, J.Y.; Wang, C.F.; Lu, H.F.; Fu, D.W.; et al. Structural Phase Transition Drives Outright Photoluminescence Quenching and Dielectric Duple Bistable Switching. Inorg. Chem. Front. 2024, 11, 2290–2299. [Google Scholar] [CrossRef]
- Yang, C.S.; Tan, Y.H.; Wang, C.F.; Chen, S.P.; Wang, B.; Wen, H.R.; Tang, Y.Z. Exceptional Second Harmonic Generation Responses, Switchable Dielectric Behaviours, and Ferroelectric Property in an Adduct of Hexamethylene-Tetramine·Bisnopinic Acid. Chem. Phys. 2018, 502, 66–71. [Google Scholar] [CrossRef]
- Xu, Y.; Xu, K.; He, L.; Yin, T.J.; Mu, J.; Men, J.T.; Zhang, W.; Ye, Q. Influence of Pd(II) Adsorption on High-Temperature Ferroelastic Phase Transition in (2-Amino-2-Thiazolinium)PbBr3. Inorg. Chem. 2023, 62, 1279–1285. [Google Scholar] [CrossRef]
- Akutagawa, T.; Koshinaka, H.; Sato, D.; Takeda, S.; Noro, S.I.; Takahashi, H.; Kumai, R.; Tokura, Y.; Nakamura, T. Ferroelectricity and Polarity Control in Solid-State Flip-Flop Supramolecular Rotators. Nat. Mater. 2009, 8, 342–347. [Google Scholar] [CrossRef]
- Shi, P.P.; Ye, Q.; Wang, H.T.; Li, Q.; Fu, D.W.; Zhang, Y. Reversible Phase Transitions and Dielectric Properties in [(CH3)4P]2[Cr2O7] and [Et4P]2[Cr3O10]. Eur. J. Inorg. Chem. 2015, 2015, 3255–3263. [Google Scholar] [CrossRef]
- Jing, C.Q.; Chai, C.Y.; Han, X.B.; Liu, C.D.; Wang, W.; Ju, T.Y.; Zhang, J.M.; Jin, M.L.; Ye, Q.; Zhang, W. Sensing Polarized Light via Switchable Rashba-Dresselhaus Spin Splitting in a Ferroelectric Semiconductor. Matter 2024, 7, 991–1001. [Google Scholar] [CrossRef]
- Xu, K.; He, L.; Wang, Y.Z.; Meng, X.; Shi, P.P.; Ye, Q. (C7H18N2)PbI4: A 2D Hybrid Perovskite Solid-State Phase Transition Material with Semiconducting Properties. Inorg. Chem. 2021, 60, 10642–10647. [Google Scholar] [CrossRef]
- Liu, W.T.; Zhang, Z.X.; Ding, K.; Fu, D.W.; Lu, H.F. Halogen Tuning Toward Dielectric Switch and Band Gap Engineering in One-Dimensional Hybrid Materials. J. Mol. Struct. 2022, 1270, 133954. [Google Scholar] [CrossRef]
- Zhang, T.; Chen, C.; Zhang, W.Y.; Ye, Q.; Fu, D.W. Semiconducting Organic-Inorganic Hybrid Material with Distinct Switchable Dielectric Phase Transition. J. Phys. Chem. C 2018, 122, 20989–20995. [Google Scholar] [CrossRef]
- Rahma, J. Synthesis and Investigation of Structural, Optical, Dielectric and Electronic Properties of a New Hybrid: C13H21N2O2. Chem. Pap. 2019, 73, 1389–1399. [Google Scholar]
- Lee, T.; Straus, D.B.; Xu, X.; Devlin, K.P.; Xie, W.; Cava, R.J. Ferromagnetic Coupling in Quasi-One-Dimensional Hybrid Iron Chloride Hexagonal Perovskites. Inorg. Chem. 2024, 63, 1543–1549. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Nomura, K.; Liu, X.; Rykov, A.I.; Jin, C.; Liu, T.; Wang, J. Structural and Magnetic Properties of 57Fe-Doped TiO2 and 57Fe/Sn-Codoped TiO2 Prepared by a Soft-Chemical Process. Eur. J. Inorg. Chem. 2016, 2016, 2131–2135. [Google Scholar] [CrossRef]
- Uher, J.M.; Steiner, M.R.; Hlina, J.A. Mono- and Disamarium Azacryptand Complexes: A Platform for Cooperative Rare-Earth Metal Chemistry. Inorg. Chem. 2022, 61, 5539–5546. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, Z.; Dong, Q.; Yu, R.; Zhu, H.; Zou, Z.; Yu, H.; Huang, K.; Jiang, X.; Xiong, X. NiCl(OH) Nanosheet Array as a High Sensitivity Electrochemical Sensor for Detecting Glucose in Human Serum and Saliva. Microchem. J. 2020, 158, 105184. [Google Scholar] [CrossRef]
- Wen, H.; Liu, G.; Qi, S.C.; Gu, C.; Yang, T.; Tan, P.; Sun, L.B. Photo-Switchable Phosphotungstic Acid Active Sites in Metal–Organic Frameworks for a Tailorable Deacetalization Reaction. J. Mater. Chem. A 2023, 11, 6869–6876. [Google Scholar] [CrossRef]
- Jameson, G.B. The Solution and Refinement of Structures with X-ray Diffraction Data from Twinned Crystals. Found. Crystallogr. 1996, 52, 43–44. [Google Scholar] [CrossRef]
- Parkin, S.; Moezzi, B.; Hope, H. XABS2: An Empirical Absorption Correction Program. J. Appl. Crystallogr. 1995, 28, 53–56. [Google Scholar] [CrossRef]
- Spackman, P.R.; Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer: A Program for Hirshfeld Surface Analysis, Visualization and Quantitative Analysis of Molecular Crystals. J. Appl. Crystallogr. 2021, 54, 1006–1011. [Google Scholar] [CrossRef]
- Jayatilaka, S.D.; Wolff, S.K.; Grimwood, D.J.; McKinnon, J.J.; Spackman, M.A. CrystalExplorer: A tool for displaying Hirshfeld surfaces and visualising intermolecular interactions in molecular crystals. Acta Crystallogr. Sect. A Found. Adv. 2006, 62, s90. [Google Scholar] [CrossRef]
- Mackenzie, C.F.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer model energies and energy frameworks: Extension to metal coordination compounds, organic salts, solvates and open-shell systems. IUCrJ 2017, 4, 575–587. [Google Scholar] [CrossRef]



| Temperature | 100 K | 293 K |
|---|---|---|
| Empirical formula | C16H26N12NiS4 | C16H26N12NiS4 |
| Formula weight | 573.44 | 573.44 |
| Crystal system | Monoclinic | Monoclinic |
| Space group | P21/n | P21/n |
| a/Å | 6.3767(3) | 6.4272(6) |
| b/Å | 10.8130(5) | 10.8858(13) |
| c/Å | 16.5638(9) | 16.6018(16) |
| α/° | 90 | 90 |
| β/° | 94.187(5) | 94.103(9) |
| γ/° | 90 | 90 |
| Volume/Å3 | 1139.04(10) | 1158.6(2) |
| Z | 2 | 2 |
| ρcalcg/cm3 | 1.672 | 1.644 |
| μ/mm−1 | 1.253 | 1.231 |
| F(000) | 596.0 | 596.0 |
| Crystal size/mm3 | 0.14 × 0.13 × 0.12 | 0.14 × 0.13 × 0.12 |
| Measured 2θ range (°) | 4.502–49.978 | 4.478–49.998 |
| Rint | 0.0262 | 0.0319 |
| GOF | 1.030 | 1.125 |
| CCDC | 2,370,103 | 2,370,104 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abuduheni, A.; Zhou, L.; Yao, Y.; Liu, Y.; Hu, H.; Liu, Z. Rotation of Hexamethylenetetramine Molecules Induces Reversible Electromagnetic Coupling Properties in Isothiocyanato-Nickel Complexes. Int. J. Mol. Sci. 2025, 26, 4050. https://doi.org/10.3390/ijms26094050
Abuduheni A, Zhou L, Yao Y, Liu Y, Hu H, Liu Z. Rotation of Hexamethylenetetramine Molecules Induces Reversible Electromagnetic Coupling Properties in Isothiocyanato-Nickel Complexes. International Journal of Molecular Sciences. 2025; 26(9):4050. https://doi.org/10.3390/ijms26094050
Chicago/Turabian StyleAbuduheni, Adila, Leilei Zhou, Yubing Yao, Yang Liu, Hongzhi Hu, and Zunqi Liu. 2025. "Rotation of Hexamethylenetetramine Molecules Induces Reversible Electromagnetic Coupling Properties in Isothiocyanato-Nickel Complexes" International Journal of Molecular Sciences 26, no. 9: 4050. https://doi.org/10.3390/ijms26094050
APA StyleAbuduheni, A., Zhou, L., Yao, Y., Liu, Y., Hu, H., & Liu, Z. (2025). Rotation of Hexamethylenetetramine Molecules Induces Reversible Electromagnetic Coupling Properties in Isothiocyanato-Nickel Complexes. International Journal of Molecular Sciences, 26(9), 4050. https://doi.org/10.3390/ijms26094050





