Overexpression of the Glycyrrhiza uralensis Phenylalanine Ammonia-Lyase Gene GuPAL1 Promotes Flavonoid Accumulation in Arabidopsis thaliana
Abstract
1. Introduction
2. Results
2.1. Identification of the PAL Gene Family in Three Medicinal Glycyrrhiza Species
2.2. Chromosomal Distribution Patterns of PAL Gene Family in Three Medicinal Glycyrrhiza Species
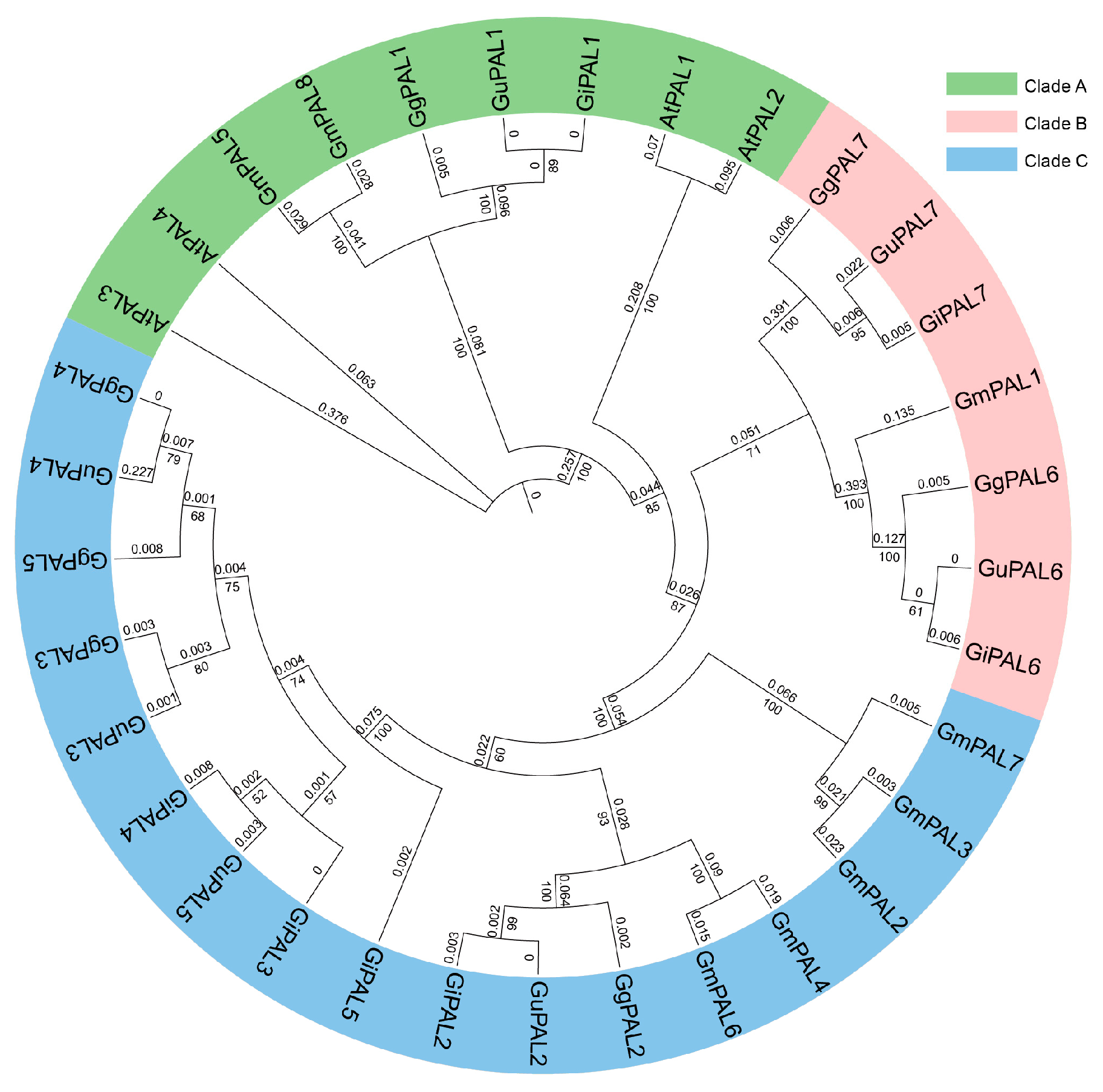
2.3. Phylogenetic Analysis of the PAL Gene Family in Three Medicinal Glycyrrhiza Species, Soybean, and A. thaliana
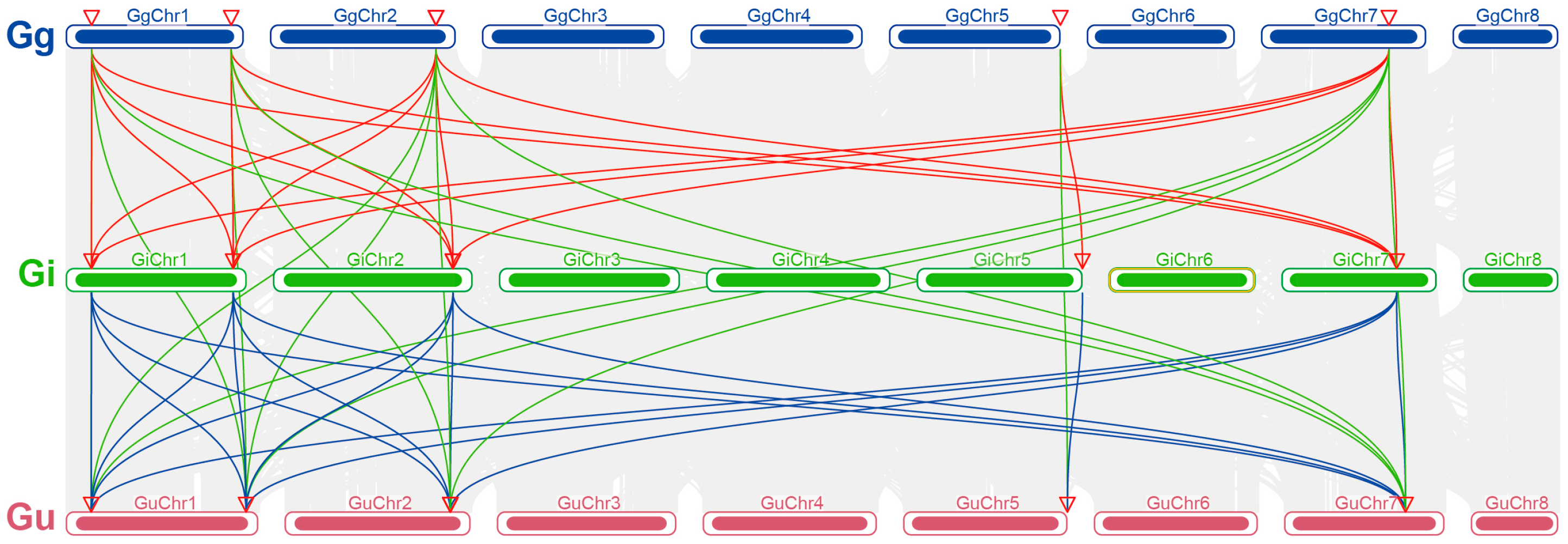
2.4. Analysis of Collinearity Relationships Among PAL Gene Families on Chromosomes in Three Medicinal Glycyrrhiza Species
2.5. Analysis of Promoter Cis-Acting Elements, Conserved Motifs, and Gene Structures of PAL Gene Family
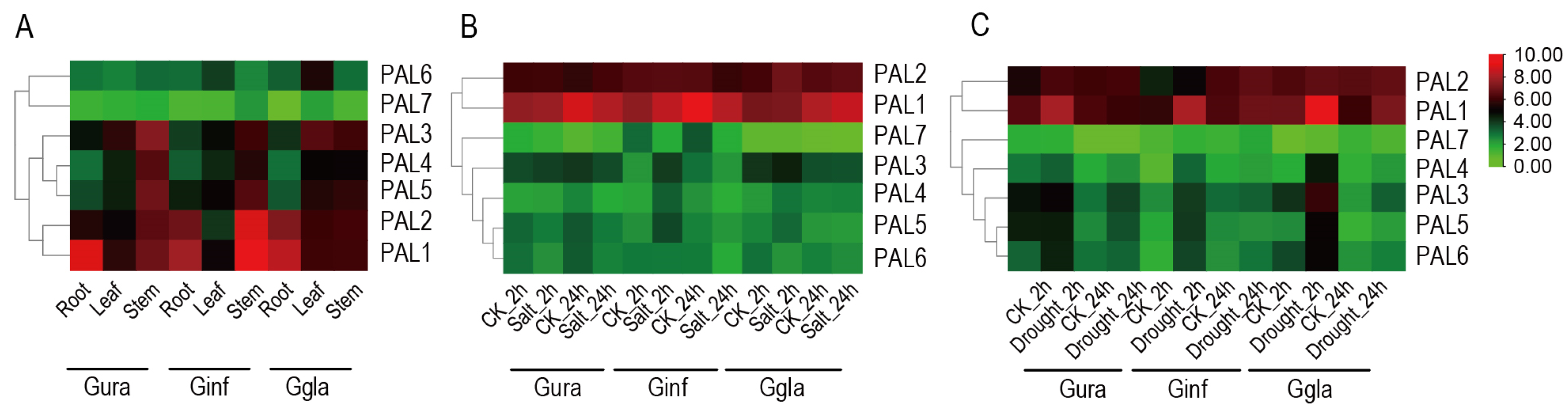
2.6. Analysis of Tissue-Specific and Stress-Induced Expression of PAL Gene Family in Three Medicinal Glycyrrhiza Species
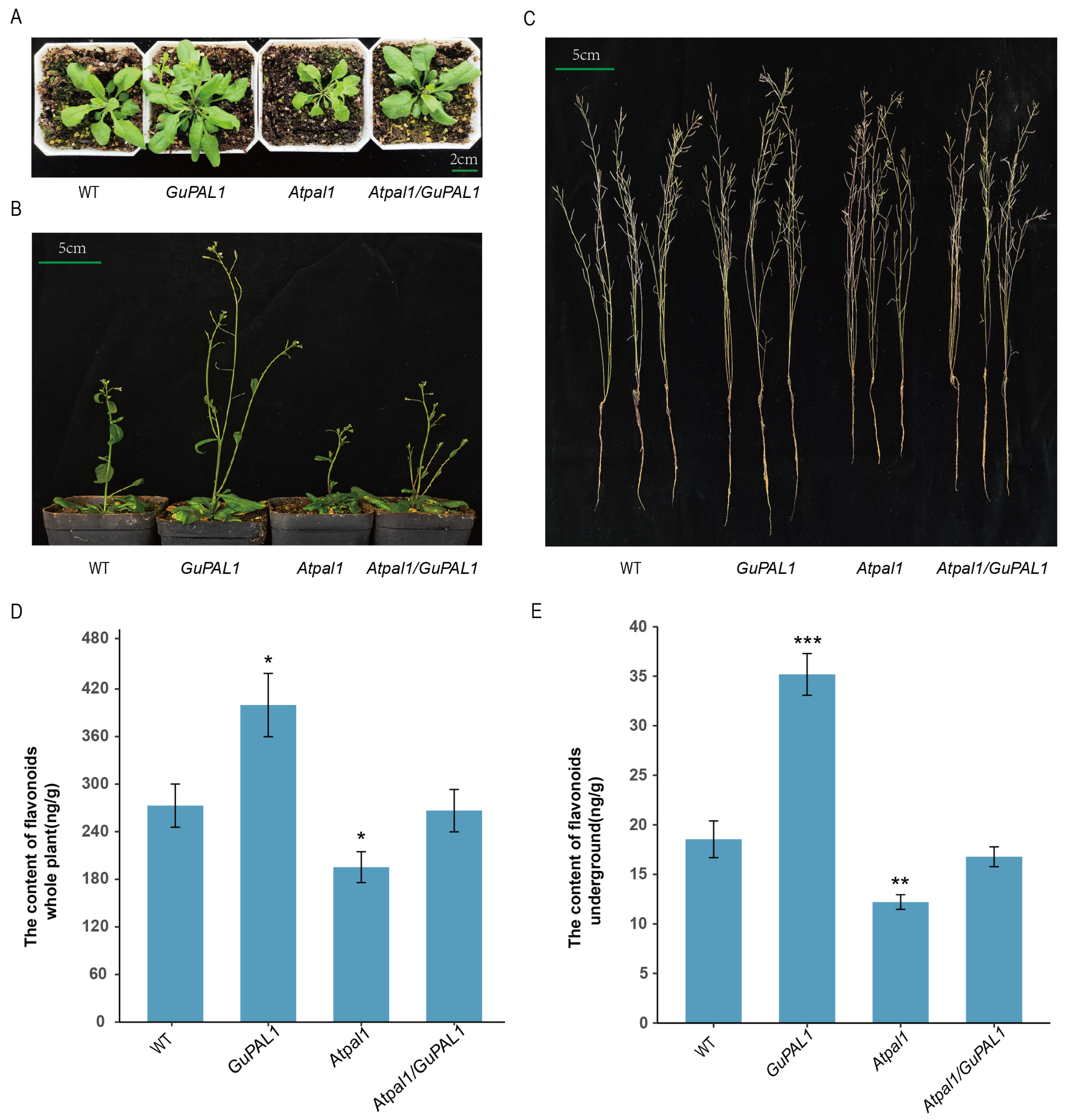
2.7. Phenotypic Analysis of Wild-Type, GuPAL1-OE, Atpal1 Mutant, and Atpal1/GuPAL1 A. thaliana Plants
2.8. Phenotypic Analysis of WT, GuPAL1-OE, Atpal1 Mutant, and Atpal1/GuPAL1 A. thaliana Plants
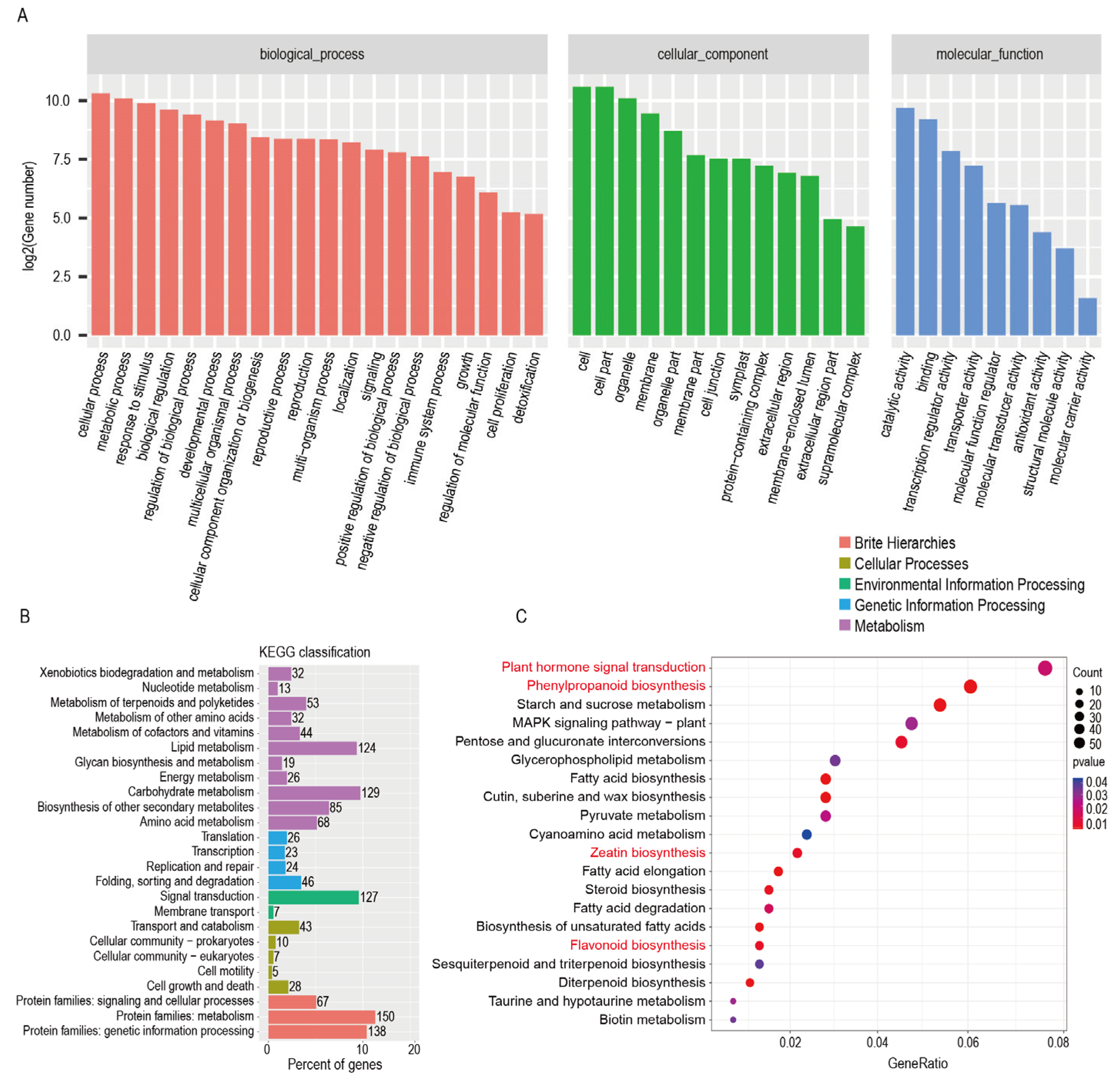
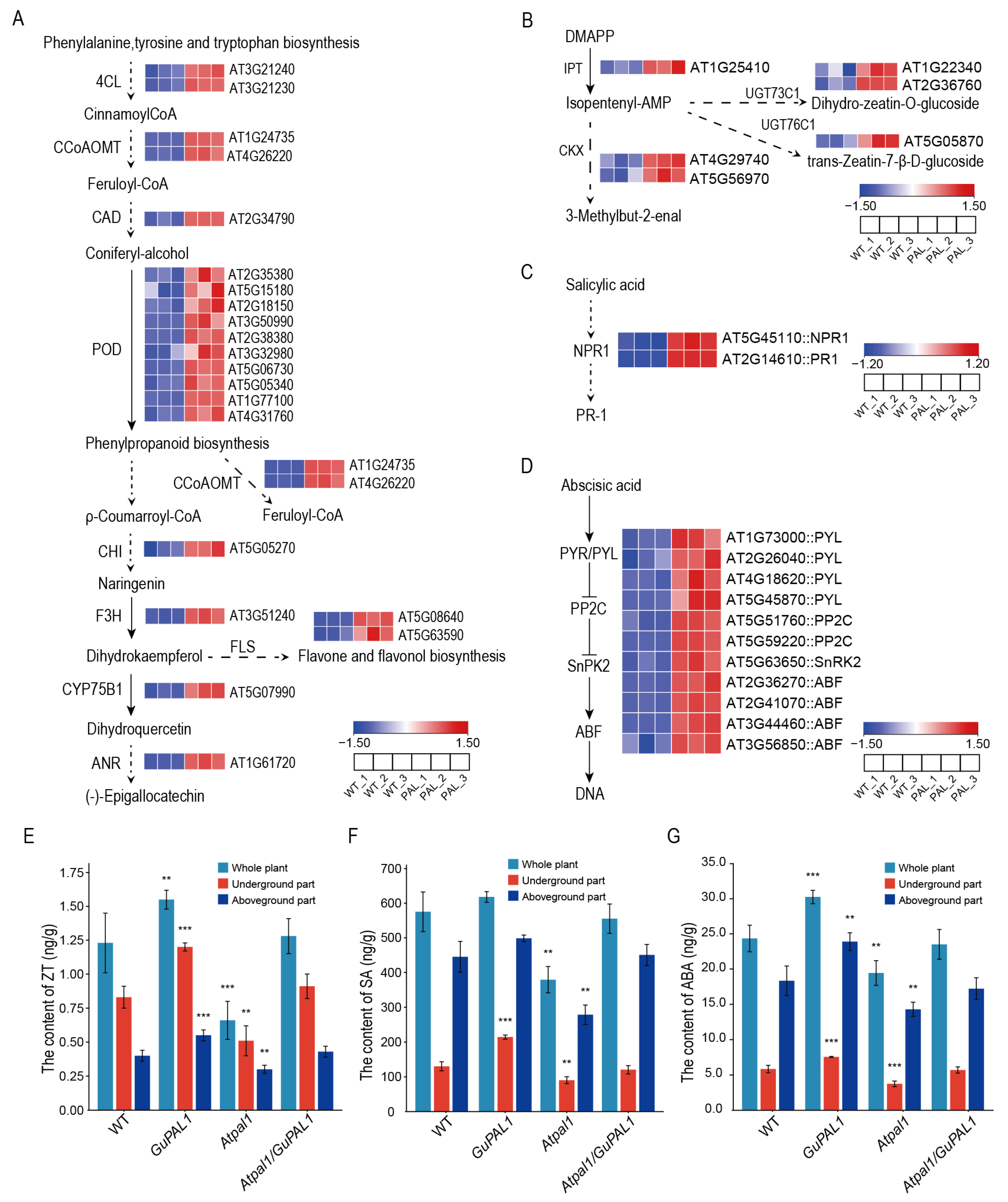
2.9. Expression Pattern of Up-Regulated Genes in the Transcriptome of GuPAL1-OE A. thaliana
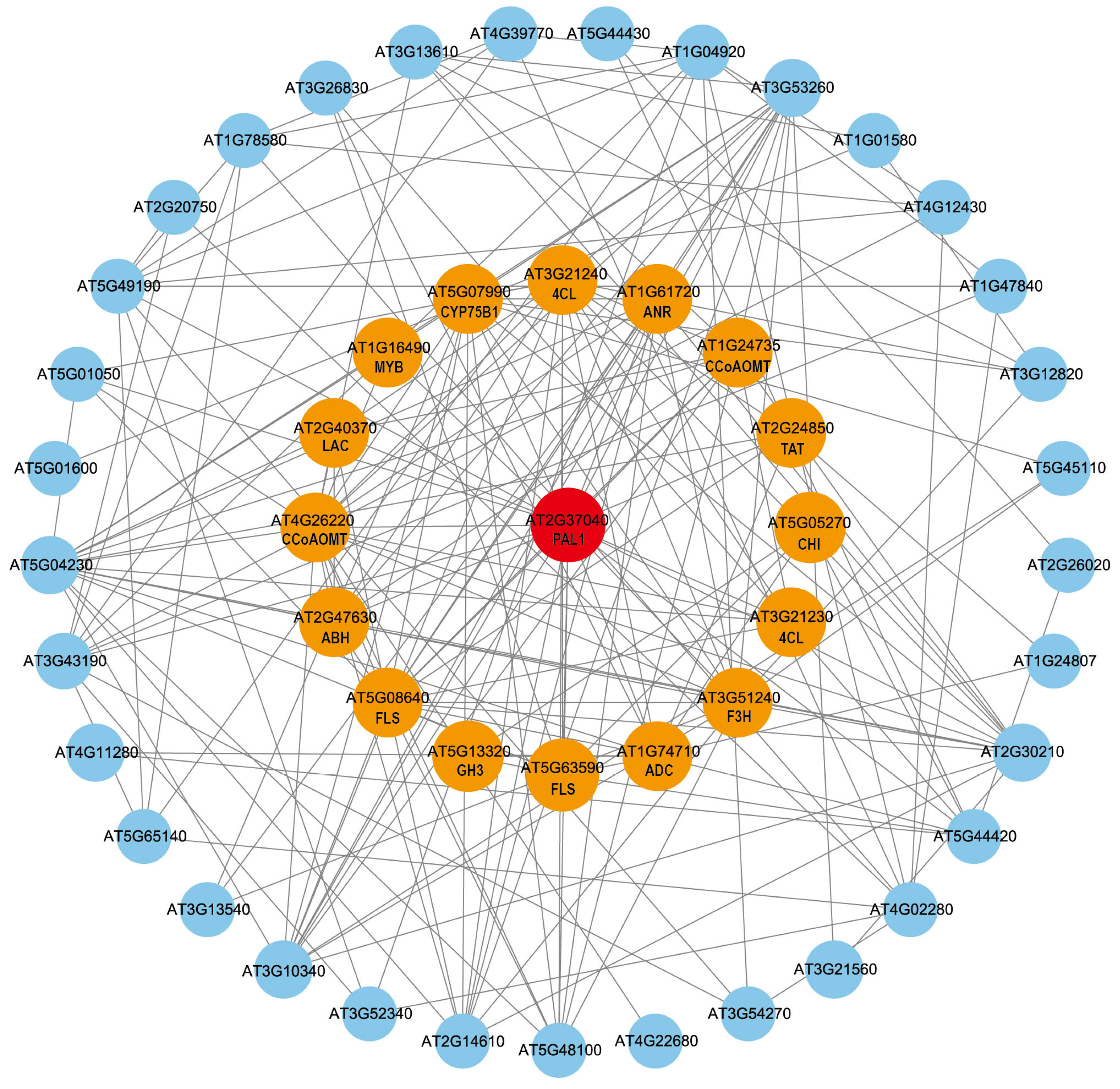
2.10. Protein–Protein Interaction (PPI) Prediction Analysis of GuPAL1
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Tissue-Specific Expression Materials and Stress Treatment in Three Medicinal Glycyrrhiza Species
4.3. Expression Pattern of PAL Family Members in Three Medicinal Glycyrrhiza Species
4.4. Construction of Plant Expression Vectors and Transformation of A. thaliana
4.5. Quantification of Flavonoids and Hormones in GuPAL1-OE A. thaliana Using LC–MS/MS
4.6. Transcriptome Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- The Pharmacopoeia Commission of the People’s Republic of China. Pharmacopoeia of the People’s Republic of China; Chemical Industry Press: Beijing, China, 2005; Volume 1, pp. 59–60.
- Kwon, Y.J.; Son, D.H.; Chung, T.H.; Lee, Y.J. A Review of the Pharmacological Efficacy and Safety of Licorice Root from Corroborative Clinical Trial Findings. J. Med. Food 2020, 23, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Searle, T.; Al–Niaimi, F.; Ali, F.R. The top 10 cosmeceuticals for facial hyperpigmentation. Dermatol. Ther. 2020, 33, e14095. [Google Scholar] [CrossRef] [PubMed]
- Alagawany, M.; Elnesr, S.S.; Farag, M.R.; Abd El–Hack, M.E.; Khafaga, A.F.; Taha, A.E.; Tiwari, R.; Yatoo, M.I.; Bhatt, P.; Marappan, G.; et al. Use of Licorice(G. glabra) Herb as a Feed Additive in Poultry: Current Knowledge and Prospects. Animals 2019, 9, 536. [Google Scholar] [CrossRef] [PubMed]
- Egamberdieva, D.; Wirth, S.; Li, L.; Abd–Allah, E.F.; Lindström, K. Microbial cooperation in the rhizosphere improves liquorice growth under salt stress. Bioengineered 2017, 8, 433–438. [Google Scholar] [CrossRef]
- Ji, X.; Liu, N.; Huang, S.; Zhang, C. A Comprehensive Review of Licorice: The Preparation; Chemical Composition; Bioactivities and Its Applications. Am. J. Chin. Med. 2024, 52, 667–716. [Google Scholar] [CrossRef]
- Zhong, C.; Chen, C.; Gao, X.; Tan, C.; Bai, H.; Ning, K. Multi–omics profiling reveals comprehensive microbe–plant–metabolite regulation patterns for medicinal plant G. uralensis Fisch. Plant Biotechnol. J. 2022, 20, 1874–1887. [Google Scholar] [CrossRef]
- Atif, R.M.; Shahid, L.; Waqas, M.; Ali, B.; Rashid, M.A.R.; Azeem, F.; Nawaz, M.A.; Wani, S.H.; Chung, G. Insights on Calcium–Dependent Protein Kinases(CPKs) Signaling for Abiotic Stress Tolerance in Plants. Int. J. Mol. Sci. 2019, 20, 5298. [Google Scholar] [CrossRef]
- Yang, Q.; Pan, J.; Shen, G.; Guo, B. Yellow light promotes the growth and accumulation of bioactive flavonoids in Epimedium pseudowushanense. J. Photochem. Photobiol. B 2019, 197, 111550. [Google Scholar] [CrossRef]
- Liu, S.; Hu, L.; Jiang, D.; Xi, W. Effect of Post–Harvest LED and UV Light Irradiation on the Accumulation of Flavonoids and Limonoids in the Segments of Newhall Navel Oranges (Citrus sinensis Osbeck). Molecules 2019, 24, 1755. [Google Scholar] [CrossRef]
- Fan, X.; He, C.; Ji, M.; Sun, X.; Luo, H.; Li, C.; Tong, H.; Zhang, W.; Sun, Z.; Chu, W. Visible light–induced deoxygenation/cyclization of salicylic acid derivatives and aryl acetylene for the synthesis of flavonoids. Chem. Commun. 2022, 58, 6348–6351. [Google Scholar] [CrossRef]
- Fu, B.; Ji, X.; Zhao, M.; He, F.; Wang, X.; Wang, Y.; Liu, P.; Niu, L. The influence of light quality on the accumulation of flavonoids in tobacco(Nicotiana tabacum L.) leaves. J. Photochem. Photobiol. B 2016, 162, 544–549. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Hu, Y.; Chen, L.; Zhang, J.; Qiao, H.; Li, W.; Zhang, K.; Fang, Y. Role of dehydration temperature on flavonoids composition and free–form volatile profile of raisins during the drying process. Food Chem. 2022, 374, 131747. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Wang, Y.; Zhao, J.; Gao, Y.; Zhang, C.; Feng, X.; Ding, X. Effect of Glomus mosseae, cadmium; and elevated air temperature on main flavonoids and phenolic acids contents in alfalfa. Environ. Sci. Pollut. Res. Int. 2023, 30, 44819–44832. [Google Scholar] [CrossRef]
- Yuan, Y.; Shuai, L.; Chen, S.; Huang, L.; Qin, S.; Yang, Z. Flavonoids and antioxidative enzymes in temperature–challenged roots of Scutellaria baicalensis Georgi. Z. Für Naturforschung C 2012, 67, 77–85. [Google Scholar] [CrossRef]
- Gouot, J.C.; Smith, J.P.; Holzapfel, B.P.; Walker, A.R.; Barril, C. Grape berry flavonoids: A review of their biochemical responses to high and extreme high temperatures. J. Exp. Bot. 2019, 70, 397–423. [Google Scholar] [CrossRef]
- Goh, H.H.; Khairudin, K.; Sukiran, N.A.; Normah, M.N.; Baharum, S.N. Metabolite profiling reveals temperature effects on the VOCs and flavonoids of different plant populations. Plant Biol. 2016, 18, 130–139. [Google Scholar] [CrossRef]
- He, Y.; Gao, Y.; Hong, H.; Geng, J.; Chen, Q.; Zhou, Y.; Zhu, Z. Megalurothrips usitatus Directly Causes the Black–Heads and Black–Tail Symptoms of Cowpea along with the Production of Insect–Resistance Flavonoids. Plants 2023, 12, 3865. [Google Scholar] [CrossRef]
- Xie, Y.; Yang, W.; Tang, F.; Chen, X.; Ren, L. Antibacterial activities of flavonoids: Structure–activity relationship and mechanism. Curr. Med. Chem. 2015, 22, 132–149. [Google Scholar] [CrossRef]
- Straney, D.; Khan, R.; Tan, R.; Bagga, S. Host recognition by pathogenic fungi through plant flavonoids. Adv. Exp. Med. Biol. 2002, 505, 9–22. [Google Scholar]
- Chen, T.; Zhou, Y.; Zhang, J.; Peng, Y.; Yang, X.; Hao, Z.; Lu, Y.; Wu, W.; Cheng, T.; Shi, J.; et al. Integrative analysis of transcriptome and proteome revealed nectary and nectar traits in the plant–pollinator interaction of Nitraria Tangutorum Bobrov. BMC Plant Biol. 2021, 21, 230. [Google Scholar] [CrossRef]
- Wang, F.; Ge, S.; Xu, X.; Xing, Y.; Du, X.; Zhang, X.; Lv, M.; Liu, J.; Zhu, Z.; Jiang, Y. Multiomics Analysis Reveals New Insights into the Apple Fruit Quality Decline under High Nitrogen Conditions. J. Agric. Food Chem. 2021, 69, 5559–5572. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.H.; Jaafar, H.Z. The relationship of nitrogen and C/N ratio with secondary metabolites levels and antioxidant activities in three varieties of Malaysian kacip Fatimah(Labisia pumila Blume). Molecules 2011, 16, 5514–5526. [Google Scholar] [CrossRef] [PubMed]
- Morales–Quintana, L.; Ramos, P. A Talk between Flavonoids and Hormones to Reorient the Growth of Gymnosperms. Int. J. Mol. Sci. 2021, 22, 12630. [Google Scholar] [CrossRef]
- Peer, W.A.; Murphy, A.S. Flavonoids and auxin transport: Modulators or regulators? Trends Plant Sci. 2007, 12, 556–563. [Google Scholar] [CrossRef]
- Sun, H.; Cui, H.; Zhang, J.; Kang, J.; Wang, Z.; Li, M.; Yi, F.; Yang, Q.; Long, R. Gibberellins Inhibit Flavonoid Biosynthesis and Promote Nitrogen Metabolism in Medicago truncatula. Int. J. Mol. Sci. 2021, 22, 9291. [Google Scholar] [CrossRef]
- Li, J.; Yang, Y.; Chai, M.; Ren, M.; Yuan, J.; Yang, W.; Dong, Y.; Liu, B.; Jian, Q.; Wang, S.; et al. Gibberellins modulate local auxin biosynthesis and polar auxin transport by negatively affecting flavonoid biosynthesis in the root tips of rice. Plant Sci. 2020, 298, 110545. [Google Scholar] [CrossRef]
- Araújo, W.L.; Martins, A.O.; Fernie, A.R.; Tohge, T. 2–Oxoglutarate: Linking TCA cycle function with amino acid; glucosinolate; flavonoid; alkaloid; and gibberellin biosynthesis. Front. Plant Sci. 2014, 5, 552. [Google Scholar] [CrossRef]
- Wu, B.; Li, Y.; Zhao, W.; Meng, Z.; Ji, W.; Wang, C. Transcriptomic and Lipidomic Analysis of Lipids in Forsythia suspensa. Front. Genet. 2021, 12, 758326. [Google Scholar] [CrossRef]
- Guo, J.; Zhou, X.; Wang, T.; Wang, G.; Cao, F. Regulation of flavonoid metabolism in ginkgo leaves in response to different day–night temperature combinations. Plant Physiol. Biochem. 2020, 147, 133–140. [Google Scholar] [CrossRef]
- Zhang, M.; Lu, X.; Ren, T.; Marowa, P.; Meng, C.; Wang, J.; Yang, H.; Li, C.; Zhang, L.; Xu, Z. Heterologous overexpression of Apocynum venetum flavonoids synthetase genes improves Arabidopsis thaliana salt tolerance by activating the IAA and JA biosynthesis pathways. Front. Plant Sci. 2023, 14, 1123856. [Google Scholar] [CrossRef]
- Ding, M.; He, Y.; Zhang, K.; Li, J.; Shi, Y.; Zhao, M.; Meng, Y.; Georgiev, M.I.; Zhou, M. JA–induced FtBPM3 accumulation promotes FtERF–EAR3 degradation and rutin biosynthesis in Tartary buckwheat. Plant J. 2022, 111, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Mao, Z.; Jiang, H.; Zhang, Z.; Wang, N.; Chen, X. Brassinolide inhibits flavonoid biosynthesis and red–flesh coloration via the MdBEH2.2–MdMYB60 complex in apple. J. Exp. Bot. 2021, 72, 6382–6399. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, L.; Ahammed, G.J.; Li, Z.X.; Wei, J.P.; Shen, C.; Yan, P.; Zhang, L.P.; Han, W.Y. Nitric oxide mediates brassinosteroid–induced flavonoid biosynthesis in Camellia sinensis L. J. Plant Physiol. 2017, 214, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Tan, Y.; Sun, Z.; Ren, A.; Zhu, J.; Zhao, M. Exogenous Salicylic Acid(SA) Promotes the Accumulation of Biomass and Flavonoid Content in Phellinus igniarius (Agaricomycetes). Int. J. Med. Mushrooms 2019, 21, 955–963. [Google Scholar] [CrossRef]
- Rodrigues, J.M.; Coutinho, F.S.; Dos Santos, D.S.; Vital, C.E.; Ramos, J.R.L.S.; Reis, P.B.; Oliveira, M.G.A.; Mehta, A.; Fontes, E.P.B.; Ramos, H.J.O. BiP–overexpressing soybean plants display accelerated hypersensitivity response(HR) affecting the SA–dependent sphingolipid and flavonoid pathways. Phytochemistry 2021, 185, 112704. [Google Scholar] [CrossRef]
- Gao, G.; Lv, Z.; Zhang, G.; Li, J.; Zhang, J.; He, C. An ABA–flavonoid relationship contributes to the differences in drought resistance between different sea buckthorn subspecies. Tree Physiol. 2021, 41, 744–755. [Google Scholar] [CrossRef]
- Brunetti, C.; Sebastiani, F.; Tattini, M. Review: ABA; flavonols; and the evolvability of land plants. Plant Sci. 2019, 280, 448–454. [Google Scholar] [CrossRef]
- Mattus–Araya, E.; Guajardo, J.; Herrera, R.; Moya–León, M.A. ABA Speeds Up the Progress of Color in Developing F. chiloensis Fruit through the Activation of PAL, CHS and ANS, Key Genes of the Phenylpropanoid/Flavonoid and Anthocyanin Pathways. Int. J. Mol. Sci. 2022, 23, 3854. [Google Scholar] [CrossRef]
- Han, C.; Chen, G.; Zheng, D.; Feng, N. Transcriptomic and metabolomic analyses reveal that ABA increases the salt tolerance of rice significantly correlated with jasmonic acid biosynthesis and flavonoid biosynthesis. Sci. Rep. 2023, 13, 20365. [Google Scholar] [CrossRef]
- Yu, W.; Gong, F.; Xu, H.; Zhou, X. Molecular Mechanism of Exogenous ABA to Enhance UV–B Resistance in Rhododendron chrysanthum Pall. by Modulating Flavonoid Accumulation. Int. J. Mol. Sci. 2024, 25, 5248. [Google Scholar] [CrossRef]
- Harbart, V.; Frede, K.; Fitzner, M.; Baldermann, S. Regulation of carotenoid and flavonoid biosynthetic pathways in Lactuca sativa var capitate L. in protected cultivation. Front. Plant Sci. 2023, 14, 1124750. [Google Scholar] [CrossRef] [PubMed]
- Winkel–Shirley, B. Flavonoid biosynthesis. A colorful model for genetics; biochemistry; cell biology; and biotechnology. Plant Physiol. 2001, 126, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Dixon, R.A.; Paiva, N.L. Stress–Induced Phenylpropanoid Metabolism. Plant Cell. 1995, 7, 1085–1097. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Feng, Y.; Yu, S.; Fan, Z.; Li, X.; Li, J.; Yin, H. The Flavonoid Biosynthesis Network in Plants. Int. J. Mol. Sci. 2021, 22, 12824. [Google Scholar] [CrossRef]
- Vogt, T. Phenylpropanoid biosynthesis. Mol. Plant. 2010, 3, 2–20. [Google Scholar] [CrossRef]
- Ververidis, F.; Trantas, E.; Douglas, C.; Vollmer, G.; Kretzschmar, G.; Panopoulos, N. Biotechnology of flavonoids and other phenylpropanoid–derived natural products. Part I: Chemical diversity; impacts on plant biology and human health. Biotechnol. J. 2007, 2, 1214–1234. [Google Scholar] [CrossRef]
- Koukol, J.; Conn, E.E. The metabolism of aromatic compounds in higher plants. IV. Purification and properties of the phenylalanine deaminase of Hordeum vulgare. J. Biol. Chem. 1961, 236, 2692–2698. [Google Scholar] [CrossRef]
- Tanaka, Y.; Uritani, I. Purification and properties of phenylalanine ammonia–lyase in cut–injured sweet potato. J. Biochem. 1977, 81, 963–970. [Google Scholar] [CrossRef]
- Havir, E.A. Phenylalanine ammonia–lyase: Purification and characterization from soybean cell suspension cultures. Arch. Biochem. Biophys. 1981, 211, 556–563. [Google Scholar] [CrossRef]
- Lim, H.W.; Park, S.S.; Lim, C.J. Purification and properties of phenylalanine ammonia–lyase from leaf mustard. Mol. Cells. 1997, 7, 715–720. [Google Scholar] [CrossRef]
- Gao, X.; Hu, Y.; Xu, Z.; Peng, D.; Guo, Q. Expression profiling of the phenylalanine ammonia–lyase(PAL) gene family in ginkgo biloba L. Plant Signal Behav. 2023, 18, 2271807. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Gu, M.; Lai, Z.; Fan, B.; Shi, K.; Zhou, Y.H.; Yu, J.Q.; Chen, Z. Functional analysis of the Arabidopsis PAL gene family in plant growth; development; and response to environmental stress. Plant Physiol. 2010, 153, 1526–1538. [Google Scholar] [CrossRef] [PubMed]
- Joos, H.J.; Hahlbrock, K. Phenylalanine ammonia–lyase in potato (Solanum tuberosum L.). Genomic complexity; structural comparison of two selected genes and modes of expression. Eur. J. Biochem. 1992, 204, 621–629. [Google Scholar] [CrossRef]
- Yang, Y.H.; Wang, C.J.; Li, R.F.; Zhang, Z.Y.; Yang, H.; Chu, C.Y.; Li, J.T. Overexpression of RgPAL family genes involved in phenolic biosynthesis promotes the replanting disease development in Rehmannia glutinosa. J. Plant Physiol. 2021, 257, 153339. [Google Scholar] [CrossRef]
- De Jong, F.; Hanley, S.J.; Beale, M.H.; Karp, A. Characterisation of the willow phenylalanine ammonia–lyase (PAL) gene family reveals expression differences compared with poplar. Phytochemistry 2015, 117, 90–97. [Google Scholar] [CrossRef]
- Jiang, H.; Fu, Y.; Li, C.; Chen, M.; Gu, Z.; Shan, Y.; Tan, X. Cadmium decreased superoxide anion derived from NADPH oxidase through overload of calcium in wheat seedling. Pak. J. Bot. 2020, 52, 1589–1594. [Google Scholar] [CrossRef]
- Feng, Y.; Huang, Q.; Zhang, R.; Li, J.; Luo, K.; Chen, Y.; Santalla, M. Molecular characterisation of PAL gene family reveals their role in abiotic stress response in lucerne (Medicago sativa). Crop Pasture Sci. 2022, 73, 300–311. [Google Scholar] [CrossRef]
- Mo, F.; Li, L.; Zhang, C.; Yang, C.; Chen, G.; Niu, Y.; Si, J.; Liu, T.; Sun, X.; Wang, S.; et al. Genome–wide analysis and expression profiling of the phenylalanine ammonia–lyase gene family in Solanum tuberosum. Int. J. Mol. Sci. 2022, 23, 6833. [Google Scholar] [CrossRef]
- Yang, X.; Liu, T.; Yang, R.; Fan, H.; Liu, X.; Xuan, Y.; Wang, Y.; Chen, L.; Duan, Y.; Zhu, X. Overexpression of GmPAL Genes Enhances Soybean Resistance Against Heterodera glycines. Mol. Plant-Microbe Interact. 2024, 37, 416–423. [Google Scholar] [CrossRef]
- Rohde, A.; Morreel, K.; Ralph, J.; Goeminne, G.; Hostyn, V.; De Rycke, R.; Kushnir, S.; Van Doorsselaere, J.; Joseleau, J.P.; Vuylsteke, M.; et al. Molecular phenotyping of the pal1 and pal2 mutants of Arabidopsis thaliana reveals far–reaching consequences on phenylpropanoid; amino acid; and carbohydrate metabolism. Plant Cell. 2004, 16, 2749–2771. [Google Scholar] [CrossRef]
- Olsen, K.M.; Lea, U.S.; Slimestad, R.; Verheul, M.; Lillo, C. Differential expression of four Arabidopsis PAL genes; PAL1 and PAL2 have functional specialization in abiotic environmental–triggered flavonoid synthesis. J. Plant Physiol. 2008, 165, 1491–1499. [Google Scholar] [CrossRef] [PubMed]
- Lavhale, S.G.; Kalunke, R.M.; Giri, A.P. Structural; functional and evolutionary diversity of 4–coumarate–CoA ligase in plants. Planta 2018, 248, 1063–1078. [Google Scholar] [CrossRef] [PubMed]
- Shen, G.; Pang, Y.; Wu, W.; Deng, Z.; Zhao, L.; Cao, Y.; Sun, X.; Tang, K. Cloning and characterization of a flavanone 3–hydroxylase gene from Ginkgo biloba. Biosci. Rep. 2006, 26, 19–29. [Google Scholar] [CrossRef]
- Zhang, C.; Guo, Q.; Liu, Y.; Liu, H.; Wang, F.; Jia, C. Molecular cloning and functional analysis of a flavanone 3–hydroxylase gene from blueberry. J. Hortic. Sci. Biotechnol. 2016, 92, 57–64. [Google Scholar] [CrossRef]
- Wang, L.; Lui, A.C.W.; Lam, P.Y.; Liu, G.; Godwin, I.D.; Lo, C. Transgenic expression of flavanone 3–hydroxylase redirects flavonoid biosynthesis and alleviates anthracnose susceptibility in sorghum. Plant Biotechnol. J. 2020, 18, 2170–2172. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, Y.; Li, K.; Yang, D.; Liu, N.; Zhang, L.; Zhao, L.; Zhang, X.; Liu, Y.; Gao, L.; et al. Roles of the 2–Oxoglutarate–Dependent Dioxygenase Superfamily in the Flavonoid Pathway: A Review of the Functional Diversity of F3H, FNS I, FLS, and LDOX/ANS. Molecules 2021, 26, 6745. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant. 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Subramanian, B.; Gao, S.; Lercher, M.J.; Hu, S.; Chen, W.H. Evolview v3: A webserver for visualization; annotation; and management of phylogenetic trees. Nucleic Acids Res. 2019, 47, W270–W275. [Google Scholar] [CrossRef]
- Bailey, T.L.; Williams, N.; Misleh, C.; Li, W.W. MEME: Discovering and analyzing DNA and protein sequence motifs. Nucleic Acids Res. 2006, 34, W369–W373. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, H.; Debarry, J.D.; Tan, X.; Li, J.; Wang, X.; Lee, T.-H.; Jin, H.; Marler, B.; Guo, H.; et al. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012, 40, e49. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Cao, A.; Wang, F.; Chen, X.; Xie, S.; Shen, H.; Jin, X.; Li, H. Calcium-Dependent Protein Kinase Genes in Glycyrrhiza uralensis Appear to be Involved in Promoting the Biosynthesis of Glycyrrhizic Acid and Flavonoids under Salt Stress. Molecules 2019, 24, 1837. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Jiang, C.; Long, M.; Hu, X.; Xu, S.; Huo, H.; Shi, R.; Xu, Q.; Xie, S.; Li, Z.; et al. Overexpression of the Glycyrrhiza uralensis Phenylalanine Ammonia-Lyase Gene GuPAL1 Promotes Flavonoid Accumulation in Arabidopsis thaliana. Int. J. Mol. Sci. 2025, 26, 4073. https://doi.org/10.3390/ijms26094073
Chen X, Jiang C, Long M, Hu X, Xu S, Huo H, Shi R, Xu Q, Xie S, Li Z, et al. Overexpression of the Glycyrrhiza uralensis Phenylalanine Ammonia-Lyase Gene GuPAL1 Promotes Flavonoid Accumulation in Arabidopsis thaliana. International Journal of Molecular Sciences. 2025; 26(9):4073. https://doi.org/10.3390/ijms26094073
Chicago/Turabian StyleChen, Xifeng, Chao Jiang, Mengqian Long, Xiangxiang Hu, Shouhao Xu, Haotong Huo, Ruixin Shi, Qing Xu, Shuangquan Xie, Zihan Li, and et al. 2025. "Overexpression of the Glycyrrhiza uralensis Phenylalanine Ammonia-Lyase Gene GuPAL1 Promotes Flavonoid Accumulation in Arabidopsis thaliana" International Journal of Molecular Sciences 26, no. 9: 4073. https://doi.org/10.3390/ijms26094073
APA StyleChen, X., Jiang, C., Long, M., Hu, X., Xu, S., Huo, H., Shi, R., Xu, Q., Xie, S., Li, Z., Shen, H., Wang, F., Xiao, G., Xie, Q., Shi, S., & Li, H. (2025). Overexpression of the Glycyrrhiza uralensis Phenylalanine Ammonia-Lyase Gene GuPAL1 Promotes Flavonoid Accumulation in Arabidopsis thaliana. International Journal of Molecular Sciences, 26(9), 4073. https://doi.org/10.3390/ijms26094073





