Role of c-ABL in DENV-2 Infection and Actin Remodeling in Vero Cells
Abstract
1. Introduction
2. Results
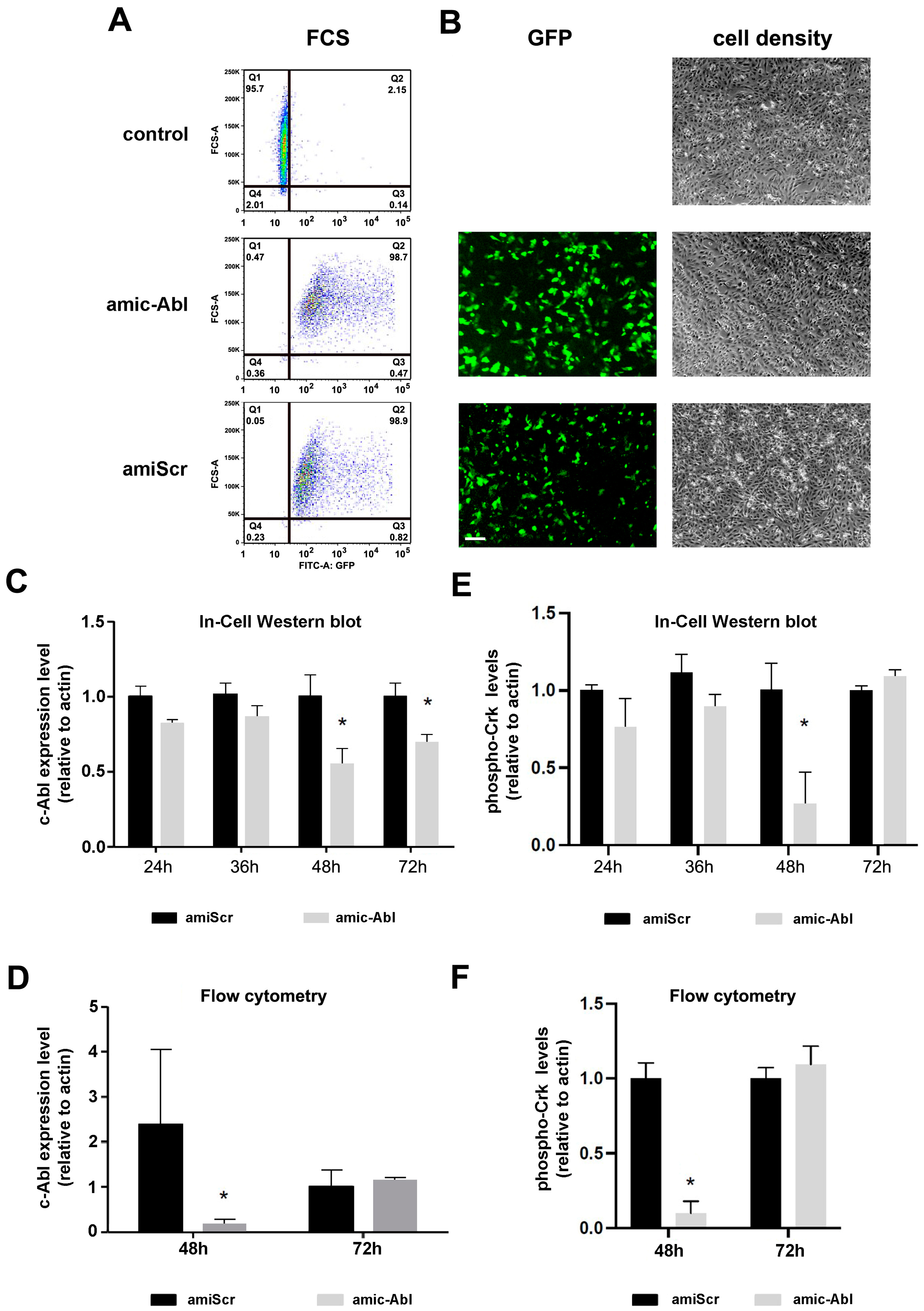
2.1. Efficient Depletion of c-ABL Using miRNA
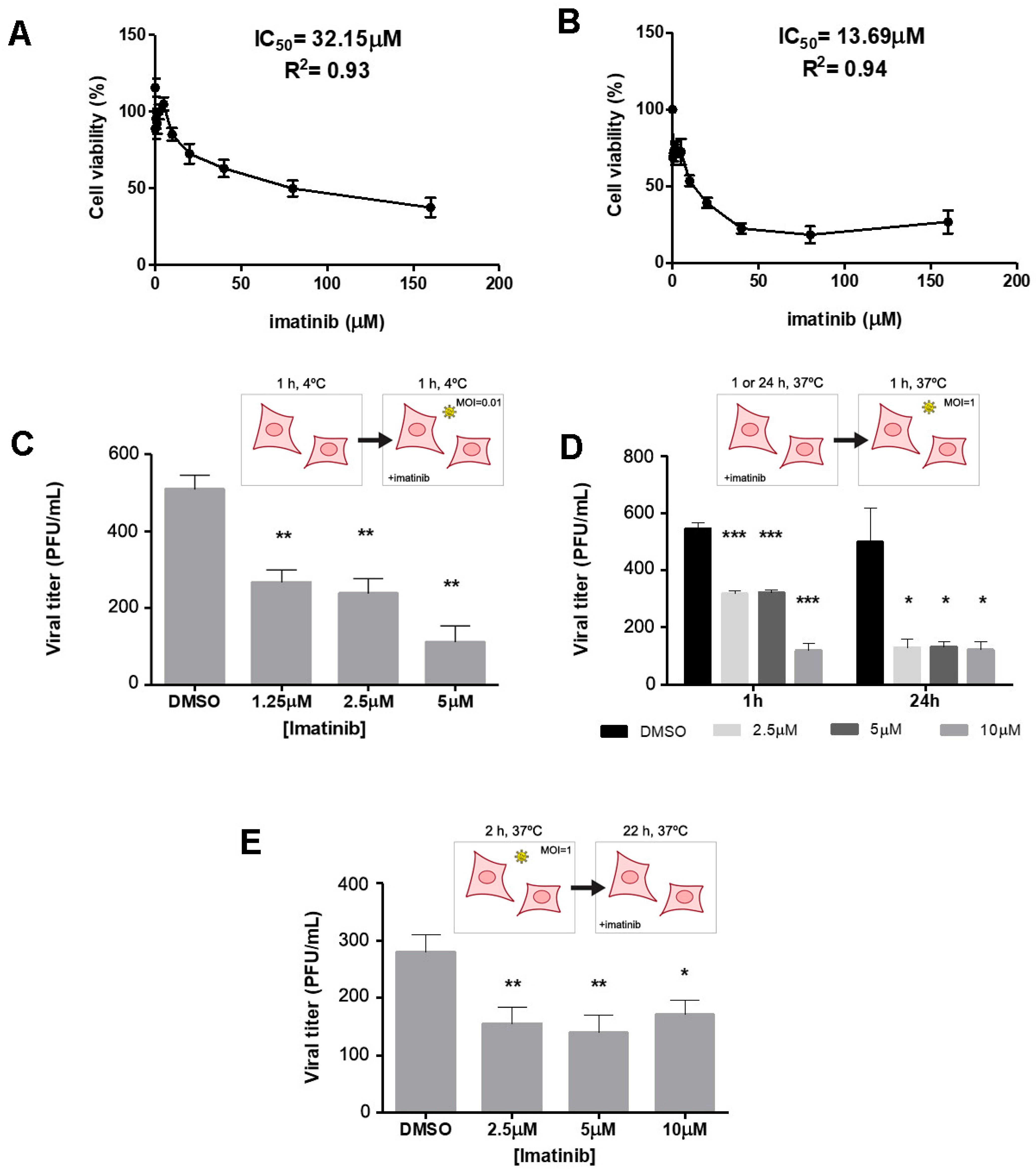
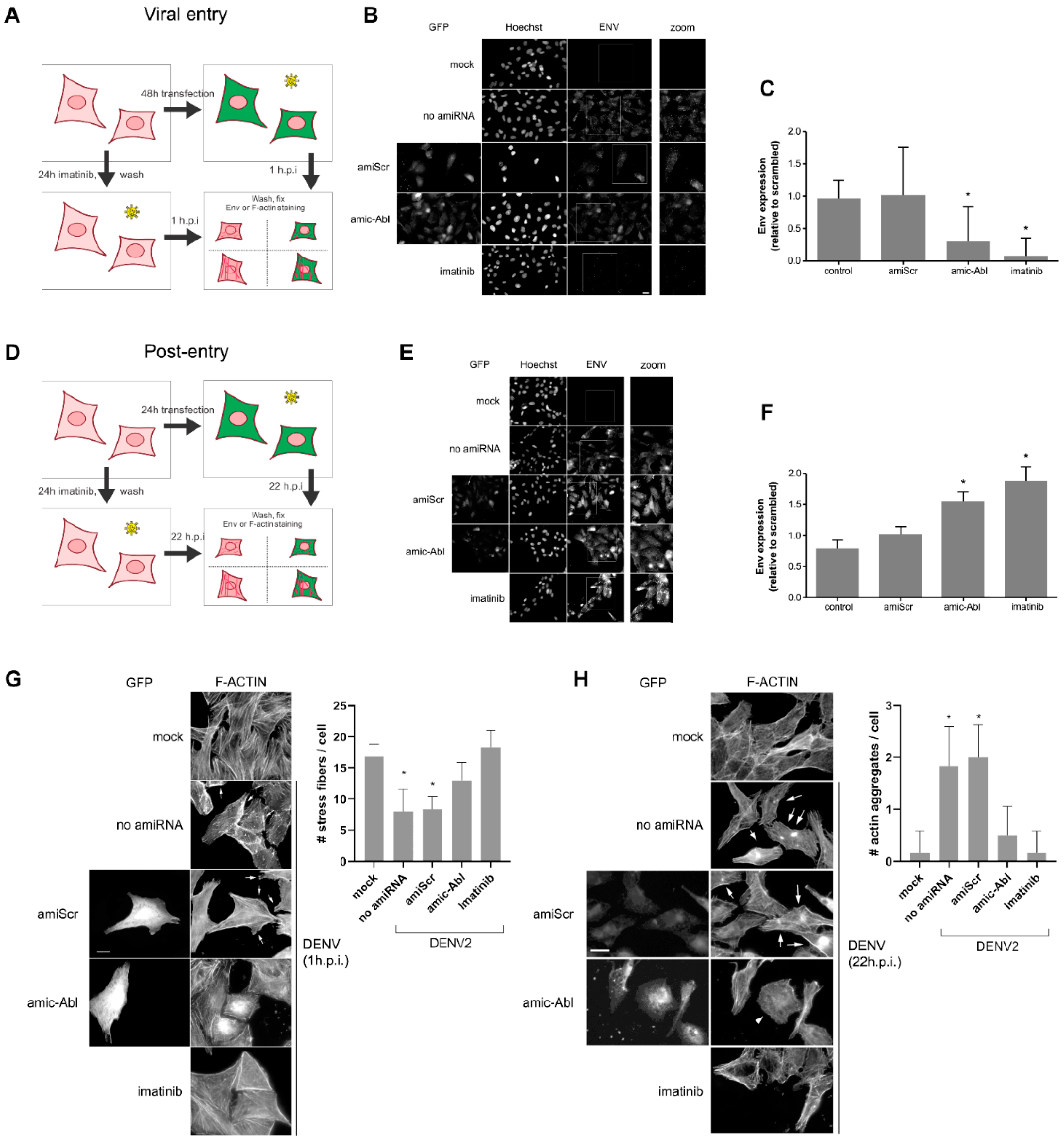
2.2. c-ABL Targeting with Imatinib or miRNA-Based Depletion Triggers DENV-2 Envelope Protein Accumulation and Actin Reorganization in Vero Cells with Different Kinetics
3. Discussion
4. Materials and Methods
4.1. Chemicals and Antibodies
4.2. Cell Lines and Virus
4.3. Plasmids, Transfection, and RNAi
4.4. Imatinib Cytotoxicity and Inhibition Treatments
4.5. Virus Binding, Entry, and Post-Entry Assays
4.6. Assessment of c-CrkII Phosphorylation by FCS
4.7. Assessment of c-ABL Silencing and c-CrkII Phosphorylation by ICWB
4.8. ENV Quantification
4.9. F-Actin Evaluation
4.10. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kularatne, S.A.; Dalugama, C. Dengue infection: Global importance, immunopathology and management. Clin. Med. 2022, 22, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.H.; Marti, C.; Diaz Perez, C.; Jackson, B.M.; Simon, A.M.; Lu, M. Epidemiology and burden of dengue fever in the United States: A systematic review. J. Travel Med. 2023, 30, taad127. [Google Scholar] [CrossRef] [PubMed]
- Renard, A.; Perez Lombardini, F.; Pacheco Zapata, M.; Porphyre, T.; Bento, A.; Suzan, G.; Roiz, D.; Roche, B.; Arnal, A. Interaction of Human Behavioral Factors Shapes the Transmission of Arboviruses by Aedes and Culex Mosquitoes. Pathogens 2023, 12, 1421. [Google Scholar] [CrossRef]
- Liang, Y.; Dai, X. The global incidence and trends of three common flavivirus infections (Dengue, yellow fever, and Zika) from 2011 to 2021. Front. Microbiol. 2024, 15, 1458166. [Google Scholar] [CrossRef]
- Roy, S.K.; Bhattacharjee, S. Dengue virus: Epidemiology, biology, and disease aetiology. Can. J. Microbiol. 2021, 67, 687–702. [Google Scholar] [CrossRef] [PubMed]
- Harapan, H.; Michie, A.; Sasmono, R.T.; Imrie, A. Dengue: A Minireview. Viruses 2020, 12, 829. [Google Scholar] [CrossRef]
- Rodríguez-Aguilar, E.D.; Martínez-Barnetche, J.; Rodríguez, M.H. Three highly variable genome regions of the four dengue virus serotypes can accurately recapitulate the CDS phylogeny. MethodsX 2022, 9, 101859. [Google Scholar] [CrossRef]
- Goh, J.Z.H.; De Hayr, L.; Khromykh, A.A.; Slonchak, A. The Flavivirus Non-Structural Protein 5 (NS5): Structure, Functions, and Targeting for Development of Vaccines and Therapeutics. Vaccines 2024, 12, 865. [Google Scholar] [CrossRef]
- Dash, M.K.; Samal, S.; Rout, S.; Behera, C.K.; Sahu, M.C.; Das, B. Immunomodulation in dengue: Towards deciphering dengue severity markers. Cell Commun. Signal. 2024, 22, 451. [Google Scholar] [CrossRef]
- Guzman, M.G.; Harris, E. Dengue. Lancet 2015, 385, 453–465. [Google Scholar] [CrossRef]
- Alfaro-Garcia, J.P.; Orozco-Castano, C.A.; Sanchez-Rendon, J.A.; Casanova-Yepes, H.F.; Vicente-Manzanares, M.; Gallego-Gomez, J.C. Characterization of the Temporal Dynamics of the Endothelial-Mesenchymal-like Transition Induced by Soluble Factors from Dengue Virus Infection in Microvascular Endothelial Cells. Int. J. Mol. Sci. 2025, 26, 2139. [Google Scholar] [CrossRef]
- Escudero-Flórez, M.; Torres-Hoyos, D.; Miranda-Brand, Y.; Gallego-Gómez, J.C.; Vicente-Manzanares, M. Dengue Virus Infection Alters Inter-Endothelial Junctions and Promotes Endothelial-Mesenchymal-Transition-like Changes in Human Microvascular Endothelial Cells. Viruses 2023, 15, 1437. [Google Scholar] [CrossRef] [PubMed]
- Tully, D.; Griffiths, C.L. Dengvaxia: The world’s first vaccine for prevention of secondary dengue. Ther. Adv. Vaccines Immunother. 2021, 9, 25151355211015839. [Google Scholar] [CrossRef] [PubMed]
- Yan, K.; Mao, L.; Lan, J.; Xiao, Z. Advancements in dengue vaccines: A historical overview and pro-spects for following next-generation candidates. J. Microbiol. 2025, 63, e2410018. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, M.N.; Garcia-Blanco, M.A. Targeting host factors to treat West Nile and dengue viral infections. Viruses 2014, 6, 683–708. [Google Scholar] [CrossRef]
- Brand, Y.M.; Roa-Linares, V.; Santiago-Dugarte, C.; Del Olmo, E.; López-Pérez, J.L.; Betancur-Galvis, L.; Gallego-Gómez, J.C.; Feliciano, A.S. A new host-targeted antiviral cyclolignan (SAU-22.107) for Dengue Virus infection in cell cultures. Potential action mechanisms based on cell imaging. Virus Res. 2023, 323, 198995. [Google Scholar] [CrossRef]
- Milani, A.; Basimi, P.; Agi, E.; Bolhassani, A. Pharmaceutical Approaches for Treatment of Hepatitis C virus. Curr. Pharm. Des. 2020, 26, 4304–4314. [Google Scholar] [CrossRef]
- Baek, Y.B.; Kwon, H.J.; Sharif, M.; Lim, J.; Lee, I.C.; Ryu, Y.B.; Lee, J.I.; Kim, J.S.; Lee, Y.S.; Kim, D.H.; et al. Therapeutic strategy targeting host lipolysis limits infection by SARS-CoV-2 and influenza A virus. Signal Transduct. Target. Ther. 2022, 7, 367. [Google Scholar] [CrossRef]
- Salasc, F.; Lahlali, T.; Laurent, E.; Rosa-Calatrava, M.; Pizzorno, A. Treatments for COVID-19: Lessons from 2020 and new therapeutic options. Curr. Opin. Pharmacol. 2022, 62, 43–59. [Google Scholar] [CrossRef]
- Naik, R.R.; Shakya, A.K.; Aladwan, S.M.; El-Tanani, M. Kinase Inhibitors as Potential Therapeutic Agents in the Treatment of COVID-19. Front. Pharmacol. 2022, 13, 806568. [Google Scholar] [CrossRef]
- Rio-Berge, C.; Cong, Y.; Reggiori, F. Getting on the right track: Interactions between viruses and the cytoskeletal motor proteins. Traffic 2023, 24, 114–130. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Neptune, E.; Cui, H. Targeting ACE2 for COVID-19 Therapy: Opportunities and Challenges. Am. J. Respir. Cell Mol. Biol. 2021, 64, 416–425. [Google Scholar] [CrossRef]
- Braun, T.P.; Eide, C.A.; Druker, B.J. Response and Resistance to BCR-ABL1-Targeted Therapies. Cancer Cell 2020, 37, 530–542. [Google Scholar] [CrossRef] [PubMed]
- Khatri, A.; Wang, J.; Pendergast, A.M. Multifunctional Abl kinases in health and disease. J. Cell Sci. 2016, 129, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Corbi-Verge, C.; Marinelli, F.; Zafra-Ruano, A.; Ruiz-Sanz, J.; Luque, I.; Faraldo-Gomez, J.D. Two-state dynamics of the SH3-SH2 tandem of Abl kinase and the allosteric role of the N-cap. Proc. Natl. Acad. Sci. USA 2013, 110, E3372–E3380. [Google Scholar] [CrossRef]
- Colicelli, J. ABL tyrosine kinases: Evolution of function, regulation, and specificity. Sci. Signal. 2010, 3, re6. [Google Scholar] [CrossRef]
- Garcia, M.; Cooper, A.; Shi, W.; Bornmann, W.; Carrion, R.; Kalman, D.; Nabel, G.J. Productive replication of Ebola virus is regulated by the c-ABL1 tyrosine kinase. Sci. Transl. Med. 2012, 4, 123ra124. [Google Scholar] [CrossRef]
- Hou, L.; Zhao, J.; Gao, S.; Ji, T.; Song, T.; Li, Y.; Wang, J.; Geng, C.; Long, M.; Chen, J.; et al. Restriction of hepatitis B virus replication by c-ABL-induced proteasomal degradation of the viral polymerase. Sci. Adv. 2019, 5, eaau7130. [Google Scholar] [CrossRef]
- Miyamoto, D.; Takeuchi, K.; Chihara, K.; Fujieda, S.; Sada, K. Protein tyrosine kinase Abl promotes hepatitis C virus particle assembly via interaction with viral substrate activator NS5A. J. Biol. Chem. 2022, 298, 101804. [Google Scholar] [CrossRef]
- Wessler, S.; Backert, S. Abl family of tyrosine kinases and microbial pathogenesis. Int. Rev. Cell Mol. Biol. 2011, 286, 271–300. [Google Scholar] [CrossRef]
- Tegtmeyer, N.; Harrer, A.; Rottner, K.; Backert, S. CagA Induces Cortactin Y-470 Phosphorylation-Dependent Gastric Epithelial Cell Scattering via Abl, Vav2 and Rac1 Activation. Cancers 2021, 13, 4241. [Google Scholar] [CrossRef] [PubMed]
- Backert, S.; Feller, S.M.; Wessler, S. Emerging roles of Abl family tyrosine kinases in microbial pathogenesis. Trends Biochem. Sci. 2008, 33, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Cui, Y.; Bai, Y.; Fang, Y.; Gao, T.; Wang, G.; Zhu, L.; Dong, Q.; Zhang, S.; Yao, Y.; et al. The tyrosine kinase c-ABL potentiates interferon-mediated antiviral immunity by STAT1 phosphorylation. iScience 2021, 24, 102078. [Google Scholar] [CrossRef] [PubMed]
- Newsome, T.P.; Weisswange, I.; Frischknecht, F.; Way, M. Abl collaborates with Src family kinases to stimulate actin-based motility of vaccinia virus. Cell Microbiol. 2006, 8, 233–241. [Google Scholar] [CrossRef]
- Reeves, P.M.; Bommarius, B.; Lebeis, S.; McNulty, S.; Christensen, J.; Swimm, A.; Chahroudi, A.; Chavan, R.; Feinberg, M.B.; Veach, D.; et al. Disabling poxvirus pathogenesis by inhibition of Abl-family tyrosine kinases. Nat. Med. 2005, 11, 731–739. [Google Scholar] [CrossRef]
- Swimm, A.I.; Bornmann, W.; Jiang, M.; Imperiale, M.J.; Lukacher, A.E.; Kalman, D. Abl family tyrosine kinases regulate sialylated ganglioside receptors for polyomavirus. J. Virol. 2010, 84, 4243–4251. [Google Scholar] [CrossRef] [PubMed]
- Coyne, C.B.; Bergelson, J.M. Virus-induced Abl and Fyn kinase signals permit coxsackievirus entry through epithelial tight junctions. Cell 2006, 124, 119–131. [Google Scholar] [CrossRef]
- Yamauchi, S.; Takeuchi, K.; Chihara, K.; Sun, X.; Honjoh, C.; Yoshiki, H.; Hotta, H.; Sada, K. Hepatitis C Virus Particle Assembly Involves Phosphorylation of NS5A by the c-ABL Tyrosine Kinase. J. Biol. Chem. 2015, 290, 21857–21864. [Google Scholar] [CrossRef]
- Harmon, B.; Campbell, N.; Ratner, L. Role of Abl kinase and the Wave2 signaling complex in HIV-1 entry at a post-hemifusion step. PLoS Pathog. 2010, 6, e1000956. [Google Scholar] [CrossRef]
- Clark, M.J.; Miduturu, C.; Schmidt, A.G.; Zhu, X.; Pitts, J.D.; Wang, J.; Potisopon, S.; Zhang, J.; Wojciechowski, A.; Hann Chu, J.J.; et al. GNF-2 Inhibits Dengue Virus by Targeting Abl Kinases and the Viral E Protein. Cell Chem. Biol. 2016, 23, 443–452. [Google Scholar] [CrossRef]
- Chen, W.; Gao, N.; Wang, J.L.; Tian, Y.P.; Chen, Z.T.; An, J. Vimentin is required for dengue virus serotype 2 infection but microtubules are not necessary for this process. Arch. Virol. 2008, 153, 1777–1781. [Google Scholar] [CrossRef]
- Kanlaya, R.; Pattanakitsakul, S.N.; Sinchaikul, S.; Chen, S.T.; Thongboonkerd, V. Vimentin interacts with heterogeneous nuclear ribonucleoproteins and dengue nonstructural protein 1 and is important for viral replication and release. Mol. Biosyst. 2010, 6, 795–806. [Google Scholar] [CrossRef] [PubMed]
- Lei, S.; Tian, Y.P.; Xiao, W.D.; Li, S.; Rao, X.C.; Zhang, J.L.; Yang, J.; Hu, X.M.; Chen, W. ROCK is involved in vimentin phosphorylation and rearrangement induced by dengue virus. Cell Biochem. Biophys. 2013, 67, 1333–1342. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, N.; Sripada, S.; Kaur, J.; Shah, P.S.; Cecilia, D. Insights into the internalization and retrograde trafficking of Dengue 2 virus in BHK-21 cells. PLoS ONE 2011, 6, e25229. [Google Scholar] [CrossRef]
- Wang, J.L.; Zhang, J.L.; Chen, W.; Xu, X.F.; Gao, N.; Fan, D.Y.; An, J. Roles of small GTPase Rac1 in the regulation of actin cytoskeleton during dengue virus infection. PLoS Negl. Trop. Dis. 2010, 4, e809. [Google Scholar] [CrossRef]
- Zamudio-Meza, H.; Castillo-Alvarez, A.; González-Bonilla, C.; Meza, I. Cross-talk between Rac1 and Cdc42 GTPases regulates formation of filopodia required for dengue virus type-2 entry into HMEC-1 cells. J. Gen. Virol. 2009, 90, 2902–2911. [Google Scholar] [CrossRef] [PubMed]
- Boudreau, R.L.; Davidson, B.L. Generation of hairpin-based RNAi vectors for biological and therapeutic application. Methods Enzymol. 2012, 507, 275–296. [Google Scholar] [CrossRef]
- Feller, S.M.; Knudsen, B.; Hanafusa, H. c-ABL kinase regulates the protein binding activity of c-Crk. EMBO J. 1994, 13, 2341–2351. [Google Scholar] [CrossRef]
- Towatari, M.; Yanada, M.; Usui, N.; Takeuchi, J.; Sugiura, I.; Takeuchi, M.; Yagasaki, F.; Kawai, Y.; Miyawaki, S.; Ohtake, S.; et al. Combination of intensive chemotherapy and imatinib can rapidly induce high-quality complete remission for a majority of patients with newly diagnosed BCR-ABL-positive acute lymphoblastic leukemia. Blood 2004, 104, 3507–3512. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Novak, M.; Zegura, B.; Nunic, J.; Gajski, G.; Geric, M.; Garaj-Vrhovac, V.; Filipic, M. Assessment of the genotoxicity of the tyrosine kinase inhibitor imatinib mesylate in cultured fish and human cells. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2017, 814, 14–21. [Google Scholar] [CrossRef]
- Woodring, P.J.; Hunter, T.; Wang, J.Y. Regulation of F-actin-dependent processes by the Abl family of tyrosine kinases. J. Cell Sci. 2003, 116, 2613–2626. [Google Scholar] [CrossRef]
- Cruz-Oliveira, C.; Freire, J.M.; Conceicao, T.M.; Higa, L.M.; Castanho, M.A.; Da Poian, A.T. Receptors and routes of dengue virus entry into the host cells. FEMS Microbiol. Rev. 2015, 39, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Tanos, B.; Pendergast, A.M. Abl tyrosine kinase regulates endocytosis of the epidermal growth factor receptor. J. Biol. Chem. 2006, 281, 32714–32723. [Google Scholar] [CrossRef] [PubMed]
- Zaitseva, E.; Yang, S.T.; Melikov, K.; Pourmal, S.; Chernomordik, L.V. Dengue virus ensures its fusion in late endosomes using compartment-specific lipids. PLoS Pathog. 2010, 6, e1001131. [Google Scholar] [CrossRef] [PubMed]
- Cuartas-López, A.M.; Hernández-Cuellar, C.E.; Gallego-Gómez, J.C. Disentangling the role of PI3K/Akt, Rho GTPase and the actin cytoskeleton on dengue virus infection. Virus Res. 2018, 256, 153–165. [Google Scholar] [CrossRef]
- Cuartas-López, A.M.; Gallego-Gómez, J.C. Glycogen synthase kinase 3ß participates in late stages of Dengue virus-2 infection. Memórias Inst. Oswaldo Cruz 2020, 115, e190357. [Google Scholar] [CrossRef]
- Kreutzman, A.; Colom-Fernandez, B.; Jimenez, A.M.; Ilander, M.; Cuesta-Mateos, C.; Perez-Garcia, Y.; Arevalo, C.D.; Bruck, O.; Hakanen, H.; Saarela, J.; et al. Dasatinib Reversibly Disrupts Endothelial Vascular Integrity by Increasing Non-Muscle Myosin II Contractility in a ROCK-Dependent Manner. Clin. Cancer Res. 2017, 23, 6697–6707. [Google Scholar] [CrossRef]
- Desmyter, J.; Melnick, J.L.; Rawls, W.E. Defectiveness of interferon production and of rubella virus interference in a line of African green monkey kidney cells (Vero). J. Virol. 1968, 2, 955–961. [Google Scholar] [CrossRef]
- Castellano, F.; Chavrier, P.; Caron, E. Actin dynamics during phagocytosis. Semin. Immunol. 2001, 13, 347–355. [Google Scholar] [CrossRef]
- Boudreau, R.L.; Martins, I.; Davidson, B.L. Artificial microRNAs as siRNA shuttles: Improved safety as compared to shRNAs in vitro and in vivo. Mol. Ther. 2009, 17, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Boudreau, R.L.; Monteys, A.M.; Davidson, B.L. Minimizing variables among hairpin-based RNAi vectors reveals the potency of shRNAs. RNA 2008, 14, 1834–1844. [Google Scholar] [CrossRef] [PubMed]
- Baer, A.; Kehn-Hall, K. Viral concentration determination through plaque assays: Using traditional and novel overlay systems. J. Vis. Exp. 2014, 93, e52065. [Google Scholar] [CrossRef]
- Talayero, V.C.; Vicente-Manzanares, M. Multi-parametric analysis of focal adhesions in bi-dimensional substrates. In The Integrin Adhesome; Vicente-Manzanares, M., Ed.; Methods Mol Biol; Springer: Berlin/Heidelberg, Germany, 2021; Volume 2217, pp. 27–37. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carreño-Flórez, G.P.; Cuartas-López, A.M.; Boudreau, R.L.; Vicente-Manzanares, M.; Gallego-Gómez, J.C. Role of c-ABL in DENV-2 Infection and Actin Remodeling in Vero Cells. Int. J. Mol. Sci. 2025, 26, 4206. https://doi.org/10.3390/ijms26094206
Carreño-Flórez GP, Cuartas-López AM, Boudreau RL, Vicente-Manzanares M, Gallego-Gómez JC. Role of c-ABL in DENV-2 Infection and Actin Remodeling in Vero Cells. International Journal of Molecular Sciences. 2025; 26(9):4206. https://doi.org/10.3390/ijms26094206
Chicago/Turabian StyleCarreño-Flórez, Grace Paola, Alexandra Milena Cuartas-López, Ryan L. Boudreau, Miguel Vicente-Manzanares, and Juan Carlos Gallego-Gómez. 2025. "Role of c-ABL in DENV-2 Infection and Actin Remodeling in Vero Cells" International Journal of Molecular Sciences 26, no. 9: 4206. https://doi.org/10.3390/ijms26094206
APA StyleCarreño-Flórez, G. P., Cuartas-López, A. M., Boudreau, R. L., Vicente-Manzanares, M., & Gallego-Gómez, J. C. (2025). Role of c-ABL in DENV-2 Infection and Actin Remodeling in Vero Cells. International Journal of Molecular Sciences, 26(9), 4206. https://doi.org/10.3390/ijms26094206






