Application of Osthol Induces a Resistance Response Against Powdery Mildew in Pumpkin Leave
Abstract
:1. Introduction
2. Results and Discussion
2.1 Results
2.1.1 Effect of osthol on powdery mildew infection of pumpkin
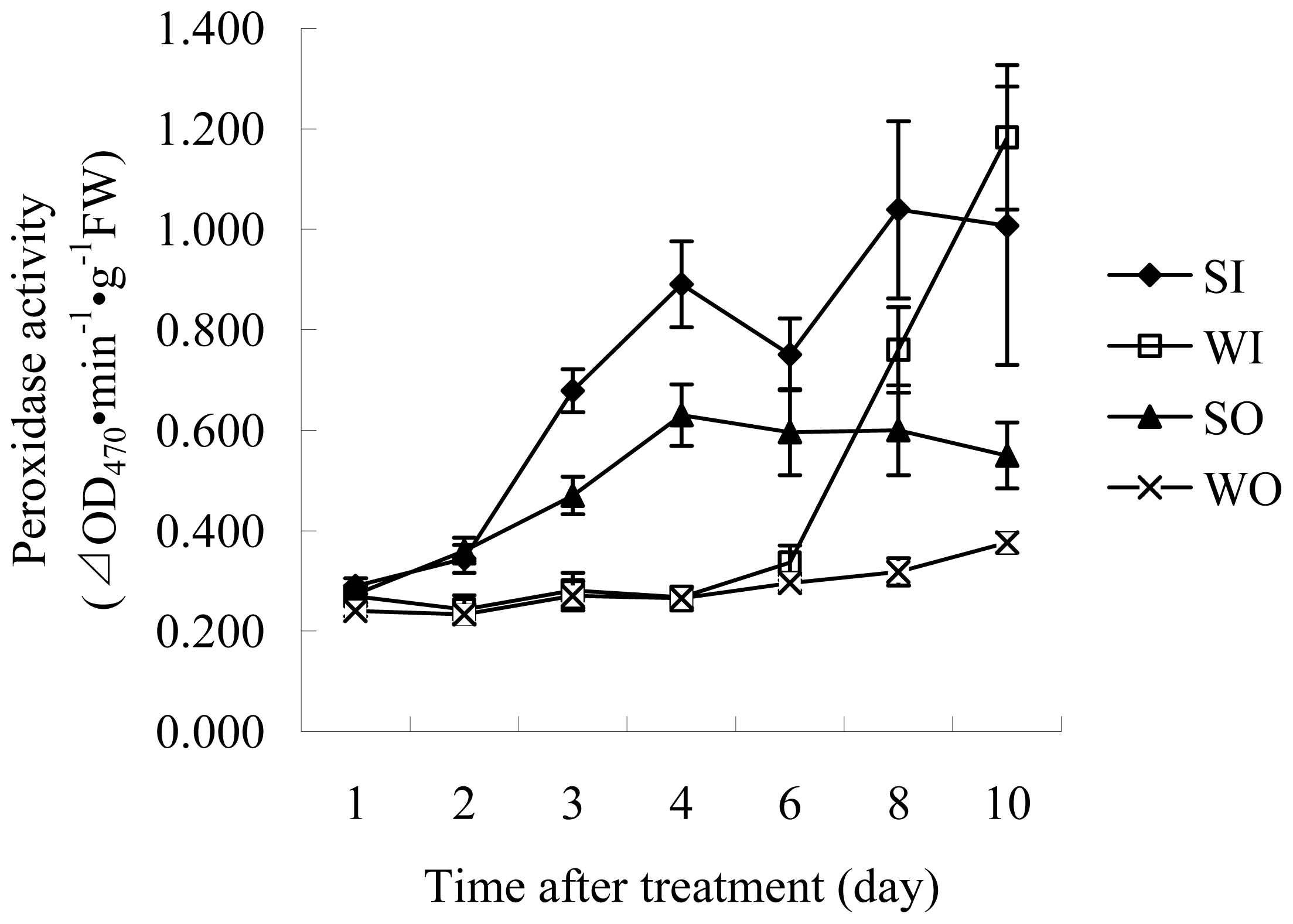
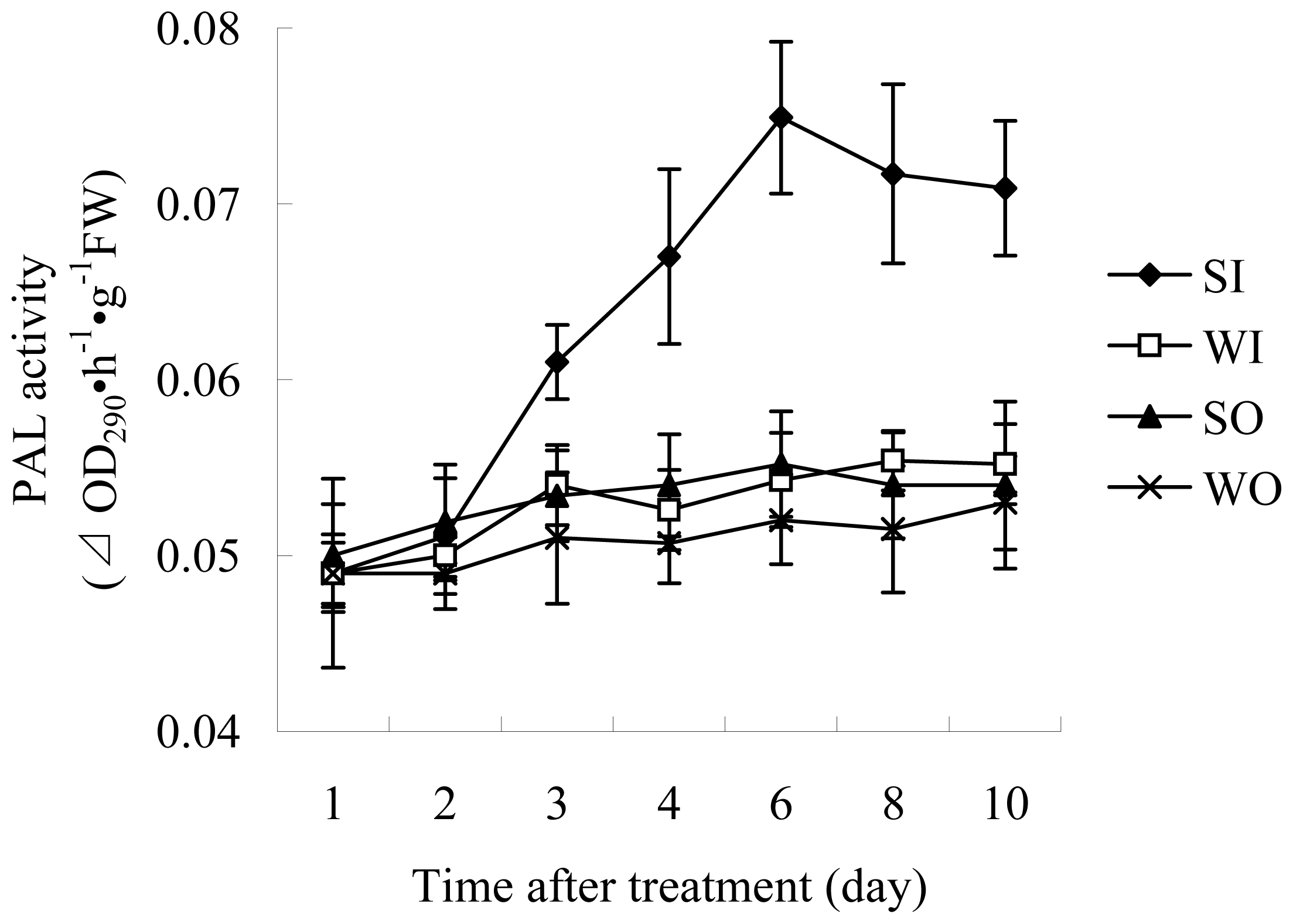
2.1.2 Enzyme Assays
2.1.3 Chitinase gene (Chitin) transcription
2.2 Discussion
3. Experimental Section
3.1 Plant material and osthol
3.2 Treatment, pathogen inoculation and disease assessment
3.3 Treatment and extraction of leaf material
3.4 Enzyme Assays
3.4.1 Chitinase activity
3.4.2 POD activity
3.4.3 PAL activity
3.5 RT-PCR


| Treatment | Mean (Disease index) a | Control efficacy b |
|---|---|---|
| Osthol (100 μg·mL−1) before inoculation | 15.56 c | 77.36 |
| Osthol (50 μg·mL−1) before inoculation | 42.22 b | 41.64 |
| Osthol (100 μg·mL−1) after inoculation | 22.63 c | 68.72 |
| Osthol (50 μg·mL−1) after inoculation | 50.78 b | 29.81 |
| Water control | 72.35 a |
Acknowledgements
References
- Rémus-Borel, W.; Menzies, J.G.; Bélanger, R.R. Silicon induces antifungal compounds in powdery mildew-infected wheat. Physiol. Mol. Plant Pathol 2005, 66, 108–115. [Google Scholar]
- Fofana, B.; Mcnally, D.J.; Labbé, C.; Boulaner, R.; Benhamou, N.; Séguin, A.; Bélanger, R.R. Milsana-induced resistance in powdery mildew-infected cucumber plant correlates with the induction of chalcone synthase and chalcone isomerase. Physiol. Mol. Plant Pathol 2002, 61, 121–132. [Google Scholar]
- Tuzun, S.; Rao, M.N.; Vogeli, U.; Schardi, C.L.; Kuc, J. Induced systemic resistance to blue mold: early induction and accumulation of β-1,3-glucanases, chitinases, and other pathogenesis-related proteins (b-proteins) in immunized tobacco. Phytopathology 1989, 79, 979–983. [Google Scholar]
- Bartnicki-Garcia, S. Cell wall chemistry, morphogeneseis, and taxonomy of fungi. Ann. Rev. Microbiol 1968, 22, 87–108. [Google Scholar]
- Broekaert, W.F.; Van Parijs, J.; Allen, A.K.; Peumans, W.J. Comparison of some molecular, enzymatic and antifungal properties of chitinases from thorn-apple, tobacco and wheat. Physiol. Mol. Plant Pathol 1988, 33, 319–331. [Google Scholar]
- Schlumbaum, A.; Mauch, F.; Vogeli, U.; Boller, T. Plant chitinases are potent inhibitors of fungal growth. Nature 1986, 324, 365–367. [Google Scholar]
- Shinshi, H.; Mohnen, D.; Meins, F. Regulation of a plant pathogenesis-related enzyme: inhibition of chitinase and chitinase mRNA accumulation in cultured tobacco tissues by auxin and cytokinin. Proc. Nat. Acad. Sci. U.S.A 1987, 84, 89–93. [Google Scholar]
- Vogeli-Lange, R.; Hansen-Gehri, A.; Boller, T.; Meins, F. Induction of the defense-related glucanohydrolase, β-1, 3-glucanase and chitinase, by tobacco mosaic virus infection of tobacco leaves. Plant Sci 1988, 54, 171–176. [Google Scholar]
- Roby, D.; Broglie, K.; Cressman, R.; Biddle, P.; Chet, I.; Broglie, R. Activation of a bean chitinase promoter in transgenic tobacco plants by phytopathogenic fungi. Plant Cell 1990, 2, 999–1007. [Google Scholar]
- Conrads-Strauch, J.; Dow, J.M.; Milligan, D.E.; Parra, R.; Daniels, M.J. Induction of hydrolytic enzymes in Brassica campestris in response to pathovars of Xanthomonas campestris. Plant Physiol 1990, 93, 238–243. [Google Scholar]
- Pharmacopoeia of the People’s Republic of China (English Edition, 1997); Chemical Industry Press: Beijing, People’s Republic of China, 1997; Volume 1, pp. 62–63.
- Ou, M. Chinese-English manual of commonly used traditional Chinese medicine; Joint Publishing Co., Ltd: Hong Kong, 1989; p. 522. [Google Scholar]
- Matsuda, H.; Tomohiro, N.; Ido, Y.; Kubo, M. Anti-allergic effects of Cnidii Monnieri fructus (dried fruits of Cnidium monnierz) and its major component, osthol. Biol. Pharm. Bull 2002, 25, 809–812. [Google Scholar]
- Liao, J.M.; Zhu, Q.A.; Lu, H.J.; Li, Q.N.; Wu, T.; Huang, L.F. Effects of total coumarins of Cnidium monnieri on bone density and biomechanics of glucocorticoids induced osteoporosis in rats. Acta Pharmacol. Sin 1997, 18, 519–521. [Google Scholar]
- Ojala, T. Biological screening of plant coumarins, University of Helsinki, Helsinki, Finland, 2001; pp. 42–45.
- Shukla, Y.N.; Srivatava, A. Phytotoxic and antimicrobial constiuents of Argyreia speciosa and Oenothera biennes. Ethnopharmacology 1986, 67, 241–245. [Google Scholar]
- Shi, Z.Q.; Shen, S.G.; Xu, L.L.; Fan, Y.J. Inhibition Mechanism of Osthol to Plant Fungus Pathogens. Chin. J. Pest. Sci 2004, 6, 28–32. [Google Scholar]
- Abenavoli, M.R.; Sorgona, A.; Muscolo, A. Morphological changes in tissue culture of Petunia hybrida in response to coumarin allelochemical. Allelopat. J 2001, 8, 171–177. [Google Scholar]
- Abenavoli, M.R.; Sorgona, A.; Albano, S.; Cacco, G. Coumarin Differentially Affects the Morphology of Different Root Types of Maize Seedlings. J. Chem. Ecol 2004, 30, 1871–1883. [Google Scholar]
- Métraux, J.P.; Burkhart, W.; Moyer, M.; Dincher, S.; Middlesteadt, W.; Williams, S.; Payne, G.; Carnes, M.; Ryals, J. Isolation of a complementary DNA encoding a chitinase with structural homology to a bifunctional lysosyme / chitinase. Proc. Nat. Acad. Sci. U.S.A 1989, 86, 896–900. [Google Scholar]
- Gozzo, F. Systemic acquired resistance in crop protection: from nature to a chemical approach. J. Agric. Food Chem 2003, 51, 4487–4503. [Google Scholar]
- Soylu, S.; Baysal, Ö.; Soylu, E.M. Induction of disease resistance by the plant activator, acibenzolar-Smethyl (ASM), against bacterial canker (Clavibacter michiganensis subsp. michiganensis) in tomato seedlings. Plant Sci 2003, 165, 1069–1075. [Google Scholar]
- Zimmerli, L.; Jakab, G.; Métraux, J.P.; Mauch-Mani, B. Potentiation of pathogen-specific defense mechanisms in Arabidopsis by β-aminobutyric acid. Proc. Nat. Acad. Sci. U.S.A 2000, 97, 12920–12925. [Google Scholar]
- Boller, T.; Gehri, A.; Mauch, F.; Vogeli, U. Chitinase in bean leaves: induction by ethylene, purification, properties, and possible function. Planta 1983, 157, 22–31. [Google Scholar]
- Nasser, W.M.; de Tapia, M.; Kauffmann, S.; Montasser-Kouhsari, S.; Burkard, G. Identification and characterization of maize pathogenesis-related proteins. Four maize PR proteins are chitinases. Plant Mol. Biol 1988, 11, 529–538. [Google Scholar]
- Hammerschmidt, R.; Nuckles, E.; Kuc, J. Association of enhanced peroxidase activity with induced systemic resistance of cucumber to Colletotrichum lagenarium. Phys. Plant Physiol 1982, 20, 73–80. [Google Scholar]
- Benhamou, N. Elicitor-induced plant defense pathways. Trends Plant Sci 1996, 1, 233–240. [Google Scholar]
- Hahlbrock, K.; Scheel, D. Physiology and molecular biology of phenylpropanoid metabolism. Ann. Rev. Plant Physiol. Plant Mol. Biol 1989, 40, 347–369. [Google Scholar]
- Zhang, C.H.; Fevereiro, P.S.; He, G.Y.; Chen, Z.J. Enhanced paclitaxel productivity and release capacity of Taxus chinensis cell suspension cultures adapted to chitosan. Plant Sci 2007, 172, 158–163. [Google Scholar]
- Hammerschmidt, R. Phenols and plant–pathogen interactions: The saga continues. Physiol. Mol. Plant Pathol 2005, 66, 77–78. [Google Scholar]
- Terry, L.A.; Joyce, D.C. Elicitors of induced disease resistance in postharvest horticultural crops: a brief review. Postharv. Biol. Tech 2004, 32, 1–14. [Google Scholar]
- Kunz, W.; Schurter, R.; Maetzke, T. The chemistry of benzothiadiazole plant activators. Pest. Sci 1997, 50, 275–282. [Google Scholar]
- Oostendorp, M.; Kunz, W.; Dietrich, B.; Staub, T. Induced disease resistance in plants by chemicals. Eur. J. Plant Pathol 2001, 107, 19–28. [Google Scholar]
- Mucharromah, E.; Kúc, J. Oxalate and phosphates induce systemic resistance against diseases caused by fungi, bacteria and viruses in cucumber. Crop Prot 1991, 10, 265–270. [Google Scholar]
- Sticher, L.; Mauch-Mani, B.; Metraux, J.P. Systemic acquired resistance. Ann. Rev. Plant Pathol 1997, 35, 235–270. [Google Scholar]
- Watanabe, T. Effects of probenazole (Oryzemater) on each stage of rice blast fungus (Pyricularia oryzae Cavara) in its life cycle. J. Pest. Sci 1977, 2, 395–404. [Google Scholar]
- Cohen, Y. Induced resistance against fungal diseases by aminobutyric acids. In Modern Fungicides and Antifungal Compounds; Lyr, H., Russell, P.E., Sisler, H.D., Eds.; Intercept: Andover, UK, 1995; pp. 461–466. [Google Scholar]
- Tosi, L.; Luigetti, R.; Zazzerini, A. Induced resistance against Plasmopara helianthi in sunflower plants by DL-betaamino-n-butyric acid. J. Phytopathol 1998, 146, 295–299. [Google Scholar]
- Langcake, P.; Cartwright, DWand; Ride, J.P. The dichlorocyclopropanes and other fungicides with indirect mode of action. In Systemische Verbindungen und antifungale Verbindungen; Lyr, H., Polter, C., Eds.; Akademie-Verlag: Berlin, 1983; pp. 199–210. [Google Scholar]
- Mauch-Mani, B. Arabidopsis-pathogen interaction: a model system for the analysis of acquired resistance. Proceedings 14th Int. Plant Protect. Cong, Jerusalem; 1999; p. 131. [Google Scholar]
- Siegrist, J.; Orober, M.; Buchenauer, H. β-Aminobutyric acid-mediated enhancement of resistance in tobacco to tobacco mosaic virus depends on the accumulation of salicylic acid. Physiol. Mol. Plant Pathol 2000, 56, 95–106. [Google Scholar]
- Shternshis, M.V.; Beljaev, A.A.; Shpatova, T.V.; Bokova, J.V.; Duzhak, A.B. Field testing of bacticide, phytoverm and chitinase for control of the raspberry midge blight in Siberia. BioControl 2002, 47, 697–706. [Google Scholar]
- Boller, T.; Metraux, J. P. Colorimentric assay for chitinase. Meth. Enzymol 1988, 161, 430–435. [Google Scholar]
- Chance, B.; Maehly, A.C. Assay of catalases and peroxidases. Meth. Enzymol 1955, 2, 764–775. [Google Scholar]
- Cheng, G.W.; Breen, P.J. Activity of phenylanaline ammonialyase (PAL) concentrations of anthocyanins and phenolics in developing strawberry fruit. J. Am. Soc. Hort. Sci 1991, 116, 865–869. [Google Scholar]
© 2007 by MDPI Reproduction is permitted for noncommercial purposes.
Share and Cite
Shi, Z.; Wang, F.; Zhou, W.; Zhang, P.; Fan, Y.J. Application of Osthol Induces a Resistance Response Against Powdery Mildew in Pumpkin Leave. Int. J. Mol. Sci. 2007, 8, 1001-1012. https://doi.org/10.3390/i8091001
Shi Z, Wang F, Zhou W, Zhang P, Fan YJ. Application of Osthol Induces a Resistance Response Against Powdery Mildew in Pumpkin Leave. International Journal of Molecular Sciences. 2007; 8(9):1001-1012. https://doi.org/10.3390/i8091001
Chicago/Turabian StyleShi, Zhiqi, Fei Wang, Wei Zhou, Peng Zhang, and Yong Jian Fan. 2007. "Application of Osthol Induces a Resistance Response Against Powdery Mildew in Pumpkin Leave" International Journal of Molecular Sciences 8, no. 9: 1001-1012. https://doi.org/10.3390/i8091001
APA StyleShi, Z., Wang, F., Zhou, W., Zhang, P., & Fan, Y. J. (2007). Application of Osthol Induces a Resistance Response Against Powdery Mildew in Pumpkin Leave. International Journal of Molecular Sciences, 8(9), 1001-1012. https://doi.org/10.3390/i8091001




