Different Magnitudes of Tensile Strain Induce Human Osteoblasts Differentiation Associated with the Activation of ERK1/2 Phosphorylation
Abstract
:1. Introduction
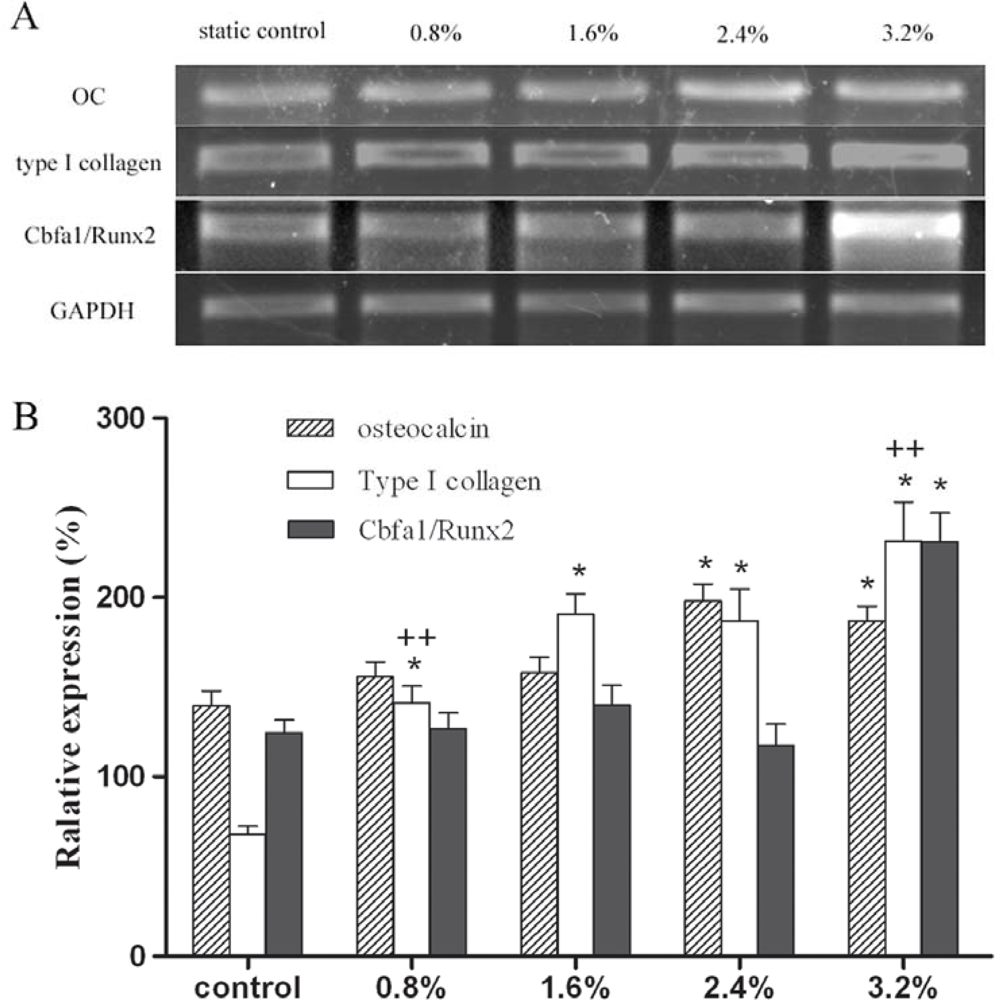
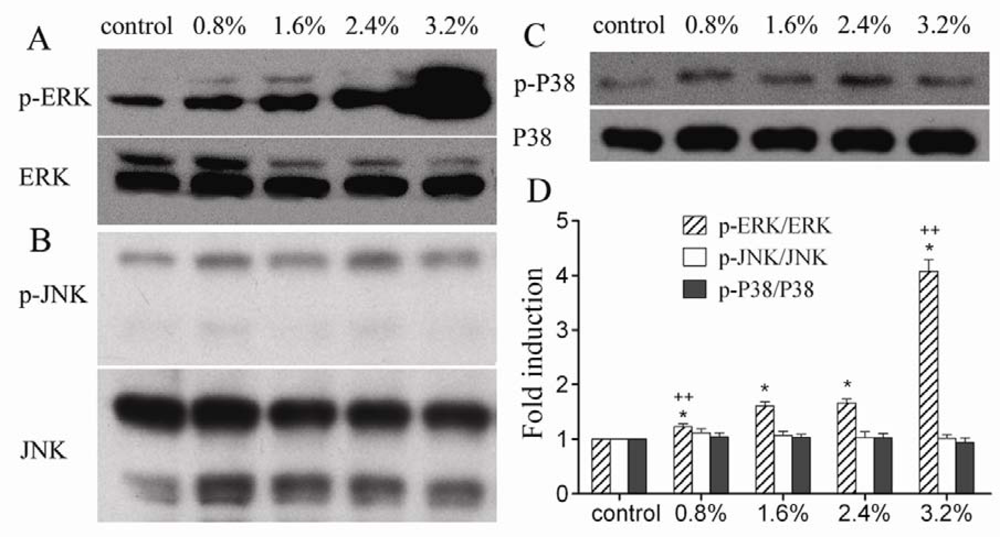
2. Results and Discussion
3. Experimental Section
3.1. Finite element (FE) model
3.2. Cell culture
3.3. Application of mechanical strain
3.4. Alkaline phosphatase (ALP) activity assay
3.5. Semi-quantitative RT-PCR
3.6. Western blot analysis of MAPK
3.7. Statistical analysis
4. Conclusions
Acknowledgments
References
- Danciu, TE; Adam, RM; Naruse, K; Freeman, MR; Hauschka, PV. Calcium regulates the PI3K-Akt pathway in stretched osteoblasts. FEBS Lett 2003, 536, 193–197. [Google Scholar]
- Weyts, FA; Bosmans, B; Niesing, R; Leeuwen, JP; Weinans, H. Mechanical control of human osteoblast apoptosis and proliferation in relation to differentiation. Calcif. Tissue Int 2003, 72, 505–512. [Google Scholar]
- Yamamoto, N; Fukuda, K; Matsushita, T; Matsukawa, M; Hara, F; Hamanishi, C. Cyclic tensile stretch stimulates the release of reactive oxygen species from osteoblast-like cells. Calcif. Tissue Int 2005, 76, 433–438. [Google Scholar]
- Fan, X; Rahnert, JA; Murphy, TC; Nanes, MS; Greenfield, EM; Rubin, J. Response to mechanical strain in an immortalized pre-osteoblast cell is dependent on ERK1/2. J. Cell Physiol 2006, 207, 454–460. [Google Scholar]
- Speirs, A; Heller, M; Duda, G; Taylor, W. Physiologically based boundary conditions in finite element modeling. J. Biomech 2007, 40, 2318–2323. [Google Scholar]
- Fritton, SP; Kenneth, JM; Rubin, CT. Quantifying the strain history of bone: spatial uniformity and self-similarity of low magnitude strains. J. Biomech 2000, 33, 317–325. [Google Scholar]
- Estok, DM; Harris, WH. A stem design change to reduce peak cement strains at the tip of cemented total hip arthroplasty. J. Arthroplasty 2000, 15, 584–589. [Google Scholar]
- Tai, CL; Lee, MS; Chen, WP; Hsieh, PH; Lee, PC; Shih, CH. Biomechanical comparison of newly designed stemless prosthesis and conventional hip prosthesis –An experimental study. Biomed. Mater. Eng 2005, 15, 239–249. [Google Scholar]
- Cowin, SC; Weinbaum, S. Strain amplification in the bone mechanosensory system. Am. J. Med. Sci 1998, 316, 184–188. [Google Scholar]
- You, L; Cowin, SC; Mitchell, BS; Mitchell, BS; Weinbaum, SA. Model for strain amplification in the actin cytoskeleton of osteocytes due to fluid drag on pericellular matrix. J. Biomech 2001, 34, 1375–1386. [Google Scholar]
- Fong, KD; Nacamuli, RP; Loboa, EG; Henderson, JH; Fang, TD; Song, HM; Cowan, CM; Warren, SM; Carter, DR; Longaker, MT. Equibiaxial tensile strain affects calvarial osteoblast biology. J. Craniofac. Surg 2003, 14, 348–355. [Google Scholar]
- Liu, XH; Zhang, XL; Luo, ZP. Strain–related collagen gene expression in human osteoblast-like cells. Cell Tissue Res 2005, 322, 331–334. [Google Scholar]
- Koike, M; Shimokawa, H; Kanno, Z; Ohya, K; Soma, K. Effects of mechanical strain on proliferation and differentiation of bone marrow stromal cell line ST2. J. Bone Miner. Metab 2005, 23, 219–225. [Google Scholar]
- Sila-Asna, M; Bunyaratvej, A; Maeda, S; Kitaguchi, H; Bunyaratavej, N. Osteoblast differentiation and bone formation gene expression in strontium-inducing bone marrow mesenchymal stem cell. Kobe J. Med. Sci 2007, 53, 25–35. [Google Scholar]
- Hakala, M; Aho, K; Aman, S; Luukkainen, R; Kauppi, M; Risteli, J. Type I collagen degradation does not diminish with RA disease duration. Ann. Rheum. Dis 2001, 60, 420–422. [Google Scholar]
- Hatton, JP; Pooran, M; Li, CF; Luzzio, C; Hughes-Fulford, M. A short pulse of mechanical force induces gene expression and growth in MC3T3-E1 osteoblasts via an ERK 1/2 pathway. J. Bone Miner. Res 2003, 18, 58–66. [Google Scholar]
- Maistrelli, GL; Fornasier, V; Binnington, A; McKenzie, K; Sessa, V; Harrington, I. Effect of stem modulus in a total hip arthroplasty model. J Bone Joint Surg. Br 1991, 73, 43–46. [Google Scholar]
- Ziros, PG; Gil, AP; Georgakopoulos, T; Habeos, I; Kletsas, D; Basdra, EK; Papavassiliou, AG. The bone specific transcriptional regulator Cbfal is a target of mechanical signals in osteoblastic cells. J. Biol. Chem 2000, 277, 23934–23941. [Google Scholar]
- Nikolovski, J; Kim, BS; Mooney, DJ. Cyclic strain inhibits switching of smooth muscle cells to an osteoblast-like phenotype. FASEB J 2003, 17, 455–457. [Google Scholar]
- Matsuda, N; Morita, N; Matsuda, K; Watanabe, M. Proliferation and differentiation of human osteoblastic cells associated with differential activation of MAP kinases in response to epidermal growth factor, hypoxia, and mechanical stress in vitro. Biochem. Biophys. Res. Commun 1998, 249, 350–354. [Google Scholar]
- Lewthwaite, JC; Bastow, ER; Lamb, KJ; Blenis, J; Wheeler-Jones, CPD; Pitsillides, AA. A specific mechanomodulatory role for p38 MAPK in embryonic joint articular surface cell MEK-ERK pathway regulation. J. Biol. Chem 2006, 281, 11011–11018. [Google Scholar]
- Khang, G; Choi, K; Kim, CS; Yang, JS; Bae, TS. A study of Korean femoral geometry. Clin. Orthop. Relat. Res 2003, 406, 116–122. [Google Scholar]
- Marshall, LM; Zmuda, JM; Chan, BK; Barrett-Connor, E; Cauley, JA; Ensrud, KE; Lang, TF; Orwoll, ES. Race and ethnic variation in proximal femur structure and BMD among older men. J. Bone Miner. Res 2008, 23, 121–130. [Google Scholar]
- Fang, D; Chiu, KY; Kemedios, ID; Yin, Q. Osteometry of the Chinese proximal femur. J. Orthop. Surg 1996, 4, 41–45. [Google Scholar]
- Chiu, KY; Fang, D. Endosteal shape of the proximal femur in Chinese. J. Orthop. Surg 1997, 5, 21–24. [Google Scholar]
- Couteau, B. Finite element modelling of the vibrational behaviour of the human femur using CT-based individualized geometrical and material properties. J. Biomech 1998, 31, 383–386. [Google Scholar]
- Radcliffe, IAJ; Prescott, P; Man, HS; Taylor, M. Determination of suitable sample sizes for multi-patient based finite element studies. Med. Eng. Phys 2007, 29, 1065–1072. [Google Scholar]
- Bergmann, G; Deuretzbacher, G; Heller, M; Graichen, F; Rohlmann, A; Strauss, J; Duda, GN. Hip contact forces and gait patterns from routine activities. J. Biomech 2001, 34, 859–871. [Google Scholar]
- Wong, AS; New, AMR; Isaacs, G; Taylor, M. Effect of bone material properties on the initial stability of a cementless hip stem: a finite element study. Proc. Inst. Mech. Eng 2005, 219, 265–275. [Google Scholar]
- Jansen, JH; Weyts, FA; Westbroek, I; Jahr, H; Chiba, H; Pols, HAP; Verhaar, JAN; van Leeuwen, JPTM; Weinans, H. Stretch-induced phosphorylation of ERK1/2 depends on differentiation stage of osteoblasts. J. Cell Biochem 2004, 93, 542–551. [Google Scholar]
- Kasten, P; Luginbuhl, R; van Griensven, M; Barkhausen, T; Krettek, C; Bohner, M; Bosch, U. Comparison of human bone marrow stromal cells seeded on calcium-deficient hydroxyapatite, beta-tricalcium phosphate and demineralized bone matrix. Biomaterials 2003, 24, 2593–2603. [Google Scholar]


| Gene | Primer sequence(Forward/Reverse) | T annealing | Cycles |
|---|---|---|---|
| GAPDH | 5′ GTTCCAATATGATTCCACCC 3′ | 52°C | 21 |
| 5′ AGGGATGATGTTCTGGAGAG 3′ | |||
| Type I collagen | 5′ ACAGCCGCTTCACCTACAGC 3′ | 52°C | 22 |
| 5′ TGCACTTTTGGTTTTTGGTCAT 3′ | |||
| Osteocalcin | 5′ GCCTTTGTGTCCAAGC 3′ | 51°C | 30 |
| 5′ GGACCCCACATCCATAG 3′ | |||
| Cbfa1/Runx2 | 5′ TACCTGAGCCAGATGACG 3′ | 58°C | 28 |
| 5′CAGTGAGGGATGAAATGC3′ |
© 2008 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/). This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhu, J.; Zhang, X.; Wang, C.; Peng, X.; Zhang, X. Different Magnitudes of Tensile Strain Induce Human Osteoblasts Differentiation Associated with the Activation of ERK1/2 Phosphorylation. Int. J. Mol. Sci. 2008, 9, 2322-2332. https://doi.org/10.3390/ijms9122322
Zhu J, Zhang X, Wang C, Peng X, Zhang X. Different Magnitudes of Tensile Strain Induce Human Osteoblasts Differentiation Associated with the Activation of ERK1/2 Phosphorylation. International Journal of Molecular Sciences. 2008; 9(12):2322-2332. https://doi.org/10.3390/ijms9122322
Chicago/Turabian StyleZhu, Junfeng, Xiaoling Zhang, Chengtao Wang, Xiaochun Peng, and Xianlong Zhang. 2008. "Different Magnitudes of Tensile Strain Induce Human Osteoblasts Differentiation Associated with the Activation of ERK1/2 Phosphorylation" International Journal of Molecular Sciences 9, no. 12: 2322-2332. https://doi.org/10.3390/ijms9122322
APA StyleZhu, J., Zhang, X., Wang, C., Peng, X., & Zhang, X. (2008). Different Magnitudes of Tensile Strain Induce Human Osteoblasts Differentiation Associated with the Activation of ERK1/2 Phosphorylation. International Journal of Molecular Sciences, 9(12), 2322-2332. https://doi.org/10.3390/ijms9122322




