Large-scale Models Reveal the Two-component Mechanics of Striated Muscle
Abstract
:1. Summary of the most important results
2. Introduction: The basic molecular event for filament sliding and force generation
3. Results
3.1. Stretch activation: Torque-increase by passive rotation of the thin filaments. Isotonic contraction: Torque-decrease by active drilling (= sliding) into the myosin cross-bridges
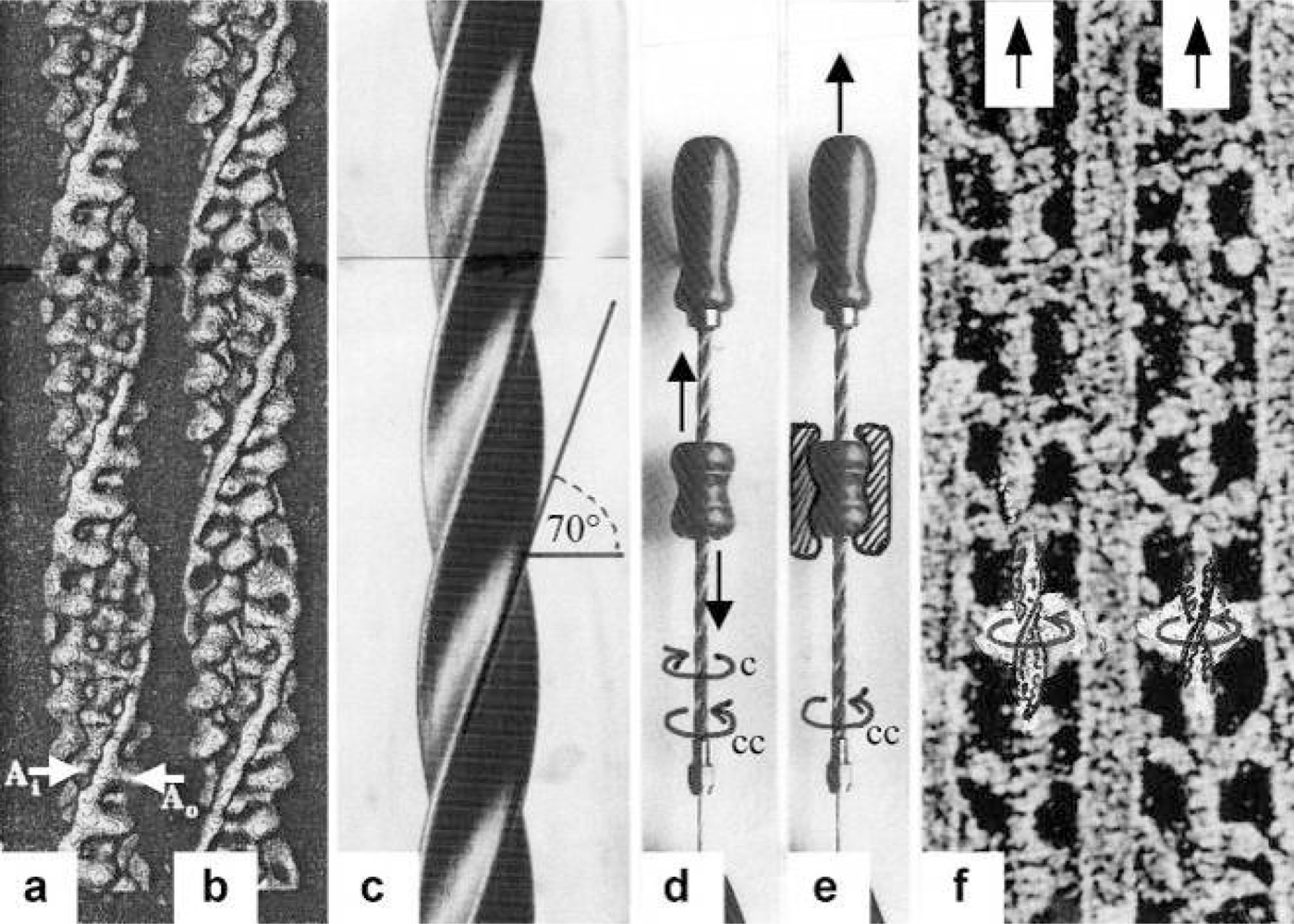
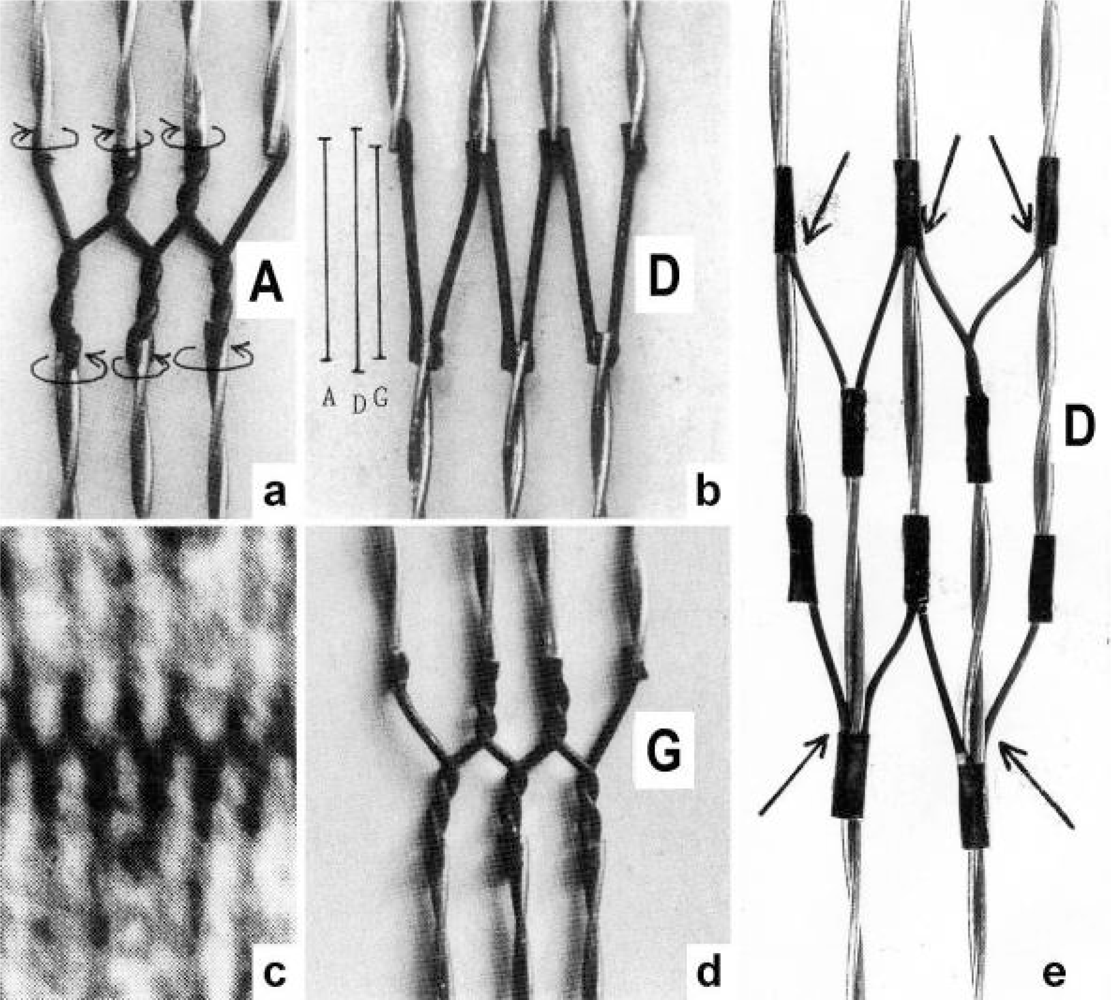
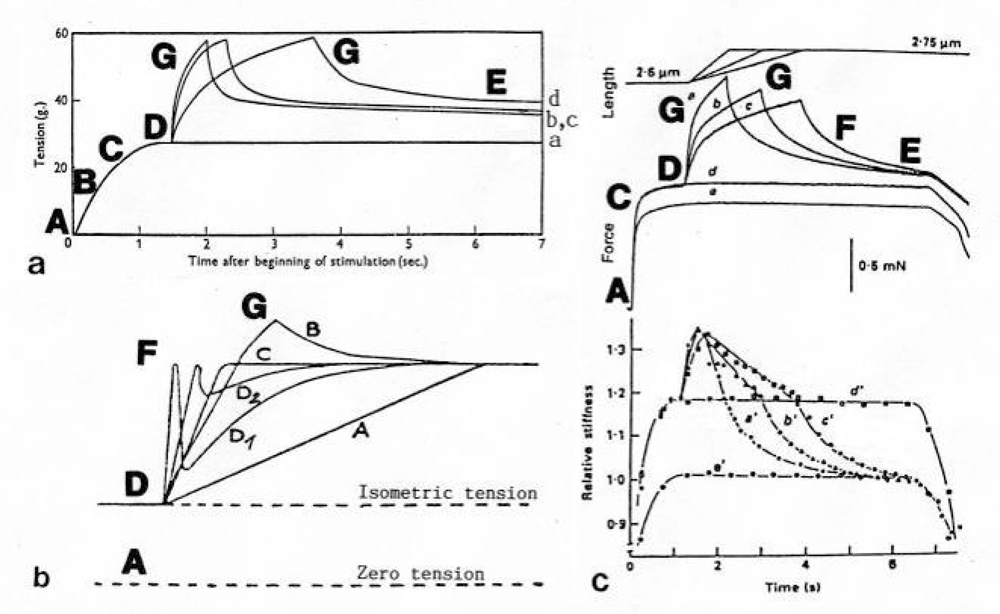
3.2. The twisting stages A to G of the four anchoring series elastic Z-filaments
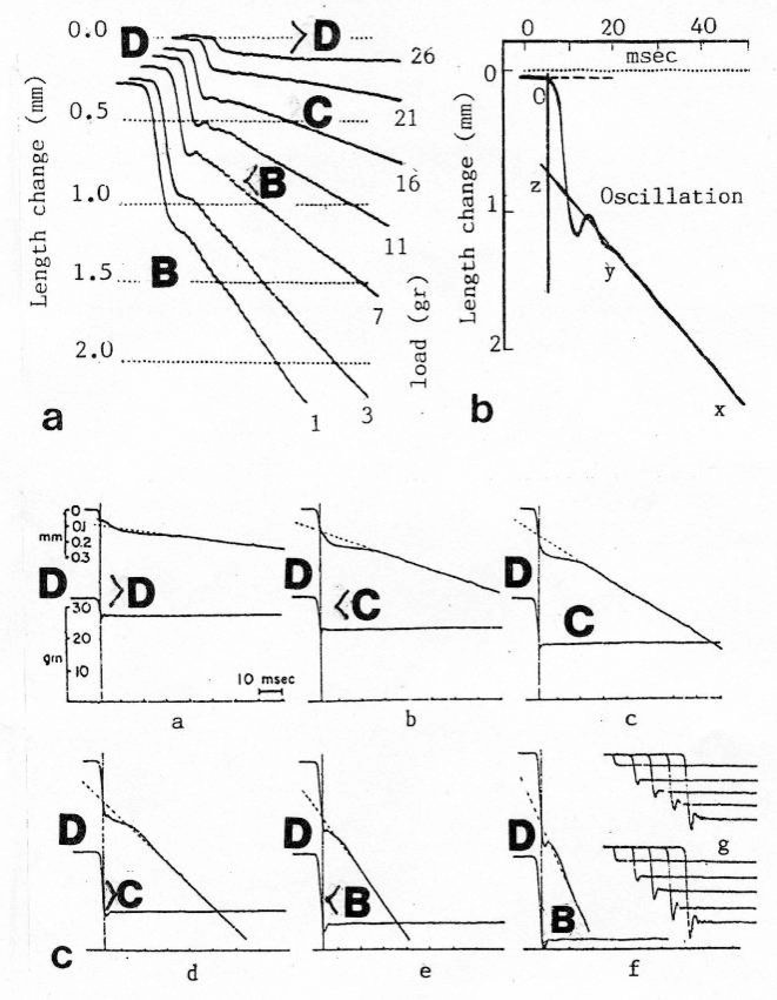
3.3. Isometric muscle force arises by stretch and unwinding the four Z-filaments from stage A to D
3.4. Z-band dynamics and the “latency-relaxation”
3.5. The Z-filaments in passive muscle
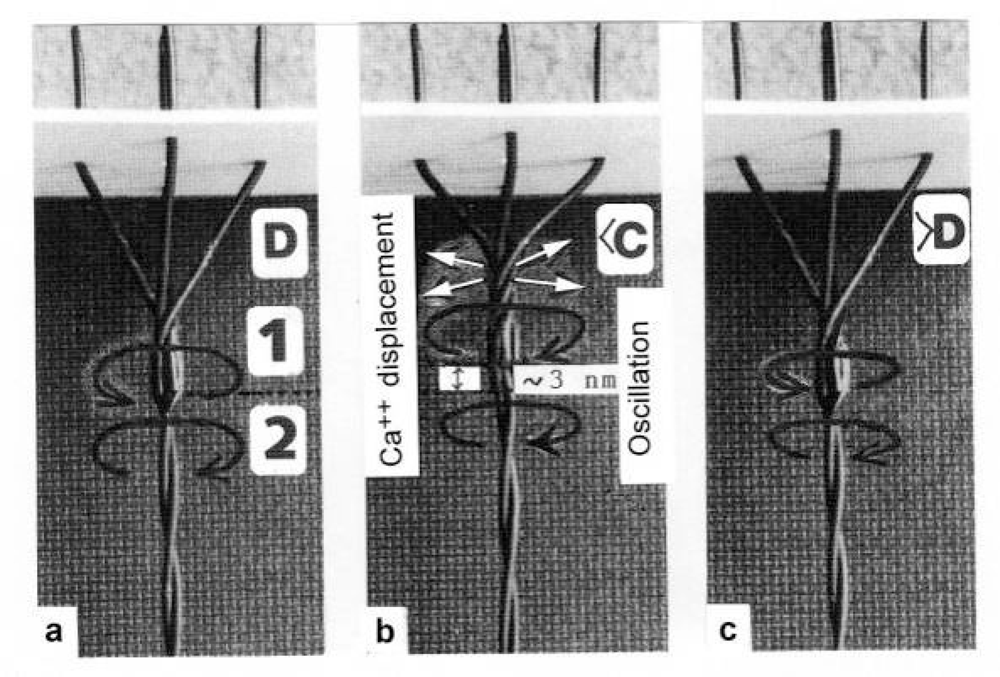
3.6. The Ca2+-activation
3.7. The binding dynamics of the Z-filaments for Ca2+
3.8. The “Fenn-effect”
3.9. The force-velocity relation
3.10. The length-tension relation and the “unexplained energy” in isometric contraction
3.11. Unexplained shortening heat is produced by the friction of drilling
3.12. Unloaded shortening velocity and sarcomere length
3.13. “Quick release”, “Slack-test” and “Force-depression”
3.14. The Huxley-Simmons phases during quick length changes
3.15. Shortening after sudden load reduction
3.16. Force depression, force enhancement and cross-bridge slipping during a length change
3.17. The Z-filaments in the region of high tension between stage D and G
Acknowledgments
References
- Holmes, KC. The molecular basis of cross-bridge function. In Sliding Filament Mechanism in Muscle Contraction; Sugi, H, Ed.; Springer: Berlin, 2005; pp. 13–23. [Google Scholar]
- Sugi, H (Ed.) Sliding Filament Mechanism in Muscle Contraction; Springer: Berlin, 2005.
- Schwyter, DH; Kron, StJ; Toyoshima, Y; Spudich, JA; Reisler, E. Subtilisin cleavage of actin inhibits in vitro sliding movement of actin filaments over myosin. J. Cell Biol 1990, 111, 465–470. [Google Scholar]
- Jarosch, R. Untersuchungen über Plasmaströmung. In Doctor Thesis; University of Vienna, 1955. [Google Scholar]
- Huxley, AF; Niedergerke, R. Structural changes in muscle during contraction. Interference microscopy of living muscle fibres. Nature 1954, 173, 971–973. [Google Scholar]
- Huxley, HE; Hanson, J. Changes in the cross-striations of muscle during contraction and stretch and their structural interpretation. Nature 1954, 173, 973–976. [Google Scholar]
- Jarosch, R. Aktiv bewegungsfähige Plasmaelemente und Chloroplastenrotation bei Characeen. Anzeig. Math.-nat. Kl. Österr. Akad. Wiss 1956, 6, 58–60. [Google Scholar]
- Jarosch, R. Plasmaströung und Chloroplastenrotation bei Characeen. Phyton (Argentina) 1956, 6, 87–107. [Google Scholar]
- Jarosch, R. Zur Mechanik der Protoplasmafibrillenbewegung. Biochim. Biophys. Acta 1957, 25, 204–205. [Google Scholar]
- Jarosch, R. Die Protoplasmafibrillen der Characeen. Protoplasma 1958, 50, 93–108. [Google Scholar]
- Jarosch, R. Die Dynamik im Characeen-Protoplasma. Phyton (Argentina) 1960, 15, 43–66. [Google Scholar]
- Hanson, J; Lowy, J. The structure of F actin and of actin filament isolated from muscle. J. Mol. Biol 1963, 6, 46–60. [Google Scholar]
- Jarosch, R. Screw-mechanical basis of protoplasmic movement. In Primitive Motile Systems in Cell Biology; Allen, RD, Kamiya, N, Eds.; Academic Press: New York, 1964; pp. 599–622. [Google Scholar]
- Jarosch, R. Dynamic behaviour of actin fibrils of Nitella based on rapid filament rotation. Biochem. Physiol. Pflanzen 1976, 170, 111–131. [Google Scholar]
- Foissner, I; Jarosch, R. The motion mechanics of Nitella filaments (cytoplasmic streaming): Their imitation in detail by screw-mechanical models. Cell Motility 1981, 1, 371–385. [Google Scholar]
- Jarosch, R. Screw-mechanical models related to cytoplasmic streaming. Protoplasma 1989, (Suppl. 1), 15–26. [Google Scholar]
- Jarosch, R. Wavelike motions of cytoskeletal fibrils and their mechanics. Acta histochemica 1992, 271–290. [Google Scholar]
- Yano, M; Yamada, T; Shimizu, H. Studies of chemomechanical conversion in artificially produced streamings. J. Biochem 1978, 84, 277–283. [Google Scholar]
- Yano, M; Yamamoto, Y; Shimizu, H. An actomyosin motor. Nature 1982, 299, 557–559. [Google Scholar]
- Tirosh, R; Oplatka, A. Active streaming against gravity in glass microcapillaries of solution containing acto-heavy meromyosin and native tropomyosin. J. Biochem 1982, 91, 1435–1440. [Google Scholar]
- Oplatka, A. Are rotors at the heart of all biological motors? Biochem. Biophys. Res. Commun 1998, 246, 301–306. [Google Scholar]
- Jarosch, R. The torsional movement of tropomyosin and the molecular mechanism of the thin filament motion. In Cell Motility: Molecules and Organization; Hatano, S, Ishikawa, H, Sato, H, Eds.; University of Tokyo Press, 1979; pp. 291–319. [Google Scholar]
- Jarosch, R. The alpha-helix, an overlooked molecular motor. Protoplasma 2005, 227, 37–46. [Google Scholar]
- Jarosch, R. The thin filament rotation model: molecular and screw-mechanical details. In Contractile Proteins in Muscle and Non-muscle Cell Systems; Alia, EE, Arena, N, Russo, MA, Eds.; Praeger: New York, 1985; pp. 239–251. [Google Scholar]
- Bacchiocchi, C; Lehrer, SS. Ca2+-induced movement of tropomyosin in skeletal muscle thin filaments observed by multi-site FRET. Biophys. J 2002, 82, 1524–1536. [Google Scholar]
- Toyoshima, YY; Kron, SJ; McNally, EM; Niebling, KR; Toyoshima, C; Spudich, JA. Myosin subfragment l is sufficient to move actin filaments in vitro. Nature 1987, 328, 536–539. [Google Scholar]
- Iwane, AH; Kitamura, K; Tokunaga, M; Yanagida, T. Myosin sub-fragment-1 is fully equipped with factors essential for motor function. Biochem. Biophys. Res. Commun 1997, 230, 76–80. [Google Scholar]
- Nishizaka, T; Yagi, T; Tanaka, Y; Ishiwata, S. Right-handed rotation of an actin filament in an in vitro motile system. Nature 1993, 361, 269–271. [Google Scholar]
- Sase, I; Miyata, H; Ishiwata, S; Kinoshita, K, Jr. Axial rotation of sliding actin filaments revealed by single-fluorophore imaging. Proc. Natl. Acad. Sci. USA 1997, 94, 5646–5650. [Google Scholar]
- Gordon, AM; Chen, Y; Liang, B; LaMadrid, M; Luo, Z; Chase, PB. Skeletal muscle regulatory proteins enhance F-actin in vitro motility. Adv. Exp. Med. Biol 1998, 453, 187–196. [Google Scholar]
- Homsher, E; Lee, DM; Morris, C; Pavlow, D; Tobacman, LS. Regulation of force and unloaded sliding speed in single thin filaments: effects of regulatory proteins and calcium. J. Physiol 2000, 524, 233–243. [Google Scholar]
- Zhao, Y; Swamy, PM; Humphries, KA; Kawai, M. The effect of partial extraction of troponin C on the elementary step of the cross-bridge cycle in rabbit psoas fibers. Biophys. J 1996, 71, 2759–2773. [Google Scholar]
- Fujita, H; Sasaki, D; Ishiwata, S; Kawai, M. Elementary steps of the cross-bridge cycle in bovine myocardium with and without regulatory proteins. Biophys. J 2002, 82, 915–928. [Google Scholar]
- Fujita, H; Ishiwata, S. Tropomyosin modulates pH dependence of isometric tension. Biophys. J 1999, 77, 1540–1546. [Google Scholar]
- Vibert, P; Craig, R; Lehman, W. Steric model for activation of muscle filaments. J. Mol. Biol 1997, 266, 8–14. [Google Scholar]
- Heuser, JE. Structure of the myosin cross-bridge lattice in insect flight muscle. J. Mol. Biol 1983, 169, 123–154. [Google Scholar]
- Jarosch, R. A simple explanation of “force enhancement” during stretch. J. Muscle Res. Cell Motil 1985, 6, 105. [Google Scholar]
- Levin, A; Wyman, J. The viscous elastic properties of muscle. Proc. R. Soc. Lond. B. Biol. Sci 1927, 101, 218–243. [Google Scholar]
- Hill, AV. The heat of shortening and the dynamic constants of muscle. Proc. R. Soc. Lond. B. Biol. Sci 1938, 126, 136–195. [Google Scholar]
- Hill, AV. The series elastic component of muscle. Proc. R. Soc. Lond. B. Biol. Sci 1950, 137, 273–280. [Google Scholar]
- Rebello, CA; Ludescher, RD. Differencial dynamic behavior of actin filaments containing tightly-bound Ca2+ or Mg2+ in the presence of myosin heads actively hydrolyzing ATP. Biochemistry 1999, 38, 13288–13295. [Google Scholar]
- Käs, J; Strey, H; Sackmann, E. Direct imaging of reptation for semiflexible actin filaments. Nature 1994, 368, 226–229. [Google Scholar]
- Tobacman, LS; Butters, CA. A new model of cooperative myosin thin filament binding. J. Biol. Chem 2000, 36, 27587–27593. [Google Scholar]
- Kushmerick, MJ; Larson, RE; Davies, RE. The chemical energetics of muscle contraction. I. Activation heat, heat of shortening and ATP utilisation for activation-relaxation processes. Proc. R. Soc. Lond. B. Biol. Sci 1969, 174, 293–313. [Google Scholar]
- Kushmerick, MJ; Davies, RE. The chemical energetics of muscle contraction. II. The chemistry, efficiency and power of maximally working sartorius muscles. Proc. R. Soc. Lond. B. Biol. Sci 1969, 174, 315–353. [Google Scholar]
- Curtin, NA; Gilbert, C; Kretzschmar, KM; Wilkie, DR. The effect of the performance of work on total energy output and metabolism during muscular contraction. J. Physiol. (Lond.) 1974, 238, 455–472. [Google Scholar]
- Rall, JA; Homsher, E; Wallner, A; Mommaerts, WFHM. A temporal dissociation of energy liberation and high energy phosphate splitting during shortening in frog skeletal muscle. J. Gen. Physiol 1976, 68, 13–27. [Google Scholar]
- Homsher, E; Irving, M; Wallner, A. High-energy phosphate metabolism and energy liberation associated with rapid shortening in frog skeletal muscle. J. Physiol. (Lond.) 1981, 321, 423–436. [Google Scholar]
- Woledge, RC; Curtin, NA; Homsher, E. Energetic Aspects of Muscle Contraction; Academic Press: London, 1985. [Google Scholar]
- Jarosch, R. Muscle force arises by actin filament rotation and torque in the Z-filaments. Biochem. Biophys. Res. Commun 2000, 270, 677–682. [Google Scholar]
- Katz, B. The relation between force and speed in muscular contraction. J. Physiol. (Lond.) 1939, 96, 45–64. [Google Scholar]
- Huxley, AF. The activation of striated muscle and its mechanical response. Proc. R. Soc. Lond. B. Biol. Sci 1971, 179, 1–27. [Google Scholar]
- Yamaguchi, M; Izumimoto, M; Robson, RM; Stromer, MH. Fine structure of wide and narrow vertebrate muscle Z-lines. A proposed model and computer simulation of Z-line architecture. J. Mol. Biol 1985, 184, 621–644. [Google Scholar]
- Landon, DW. Change in Z-disc structure with muscular contraction. J. Physiol 1970, 211, 44–45P. [Google Scholar]
- Goldstein, MA; Schroeter, JP; Sass, RL. The Z-band lattice in a slow skeletal muscle. J. Muscle Res. Cell Mot 1982, 3, 333–348. [Google Scholar]
- Goldstein, MA; Michael, LH; Schroeter, JP; Sass, RL. The Z-band lattice in skeletal muscle before, during and after tetanic contraction. J. Muscle Res. Cell Mot 1986, 7, 527–536. [Google Scholar]
- Goldstein, MA; Michael, LH; Schroeter, JP; Sass, RL. Z-band dynamics as a function of sarcomere length and the contractile state of muscle. FASEB J 1987, 1, 133–142. [Google Scholar]
- Yamaguchi, M; Fuller, GA; Klomkleaw, W; Yamono, S; Oba, T. Z-line structural diversity in frog single muscle fiber in the passive state. J. Muscle Res. Cell Mot 1999, 20, 371–381. [Google Scholar]
- Baylor, SM; Hollingworth, S. Measurement and interpretation of cytoplasmic Ca2+ signals from calcium-indicator dyes. New Physiol. Sci 2000, 15, 19–26. [Google Scholar]
- Yagi, N. An X-ray diffraction study on early structural changes in skeletal muscle contraction. Biophys. J 2003, 84, 1093–1102. [Google Scholar]
- Ramsey, RW; Street, SF. The isometric length-tension diagram of isolated skeletal muscle fibers of the frog. J. Cell. Comp. Physiol 1940, 9, 11–34. [Google Scholar]
- Hill, AV. Is relaxation an active process? Proc. R. Soc. Lond. B. Biol. Sci 1949, 136, 420–435. [Google Scholar]
- Hanson, J. Elongation of cross-striated myofibrils. Biochim. Biophys. Acta 1956, 20, 289. [Google Scholar]
- Parsons, C; Porter, RK. Muscle relaxation: Evidence for an intrafibrillar restoring force in vertebrate striated muscle. Science 1966, 153, 426. [Google Scholar]
- Gonzalez-Serratos, H. Inward spread of activation in vertebrate muscle fibres. J. Physiol 1971, 212, 777–799. [Google Scholar]
- Gonzalez-Serratos, H; Ortega, A; Valle-Aguilera, R; Chang, R. From inward spread of activation, active elongation to the effect of organic calcium channel blockers in muscle exitation-contraction coupling. In Sliding Filament Mechanism in Muscle Contraction; Sugi, H, Ed.; Springer: Berlin, 2005; pp. 249–365. [Google Scholar]
- Hill, AV. The development of the active state of muscle during the latent period. Proc. R. Soc. Lond. B. Biol. Sci 1950, 137, 320–331. [Google Scholar]
- Hill, AV. Trails and Trials in Physiology; Arnold: London, 1965. [Google Scholar]
- Knappeis, GG; Carlsen, F. The ultrastructure of the Z disc in skeletal muscle. J. Cell Biol 1962, 13, 323–335. [Google Scholar]
- Franzini-Armstrong, C; Porter, KR. The Z-disc of skeletal muscle fibrils. Z. Zellforsch. Mikroskop. Anat 1964, 61, 661. [Google Scholar]
- Reedy, MK. The structure of actin filaments and the origin of the axial periodicity in the I-substance of vertebrate striated muscle. Proc. R. Soc. Lond. B. Biol. Sci 1964, 160, 485–460. [Google Scholar]
- Huxley, AF. Muscle structure and theories of contraction. Prog. Biophys. Biophys. Chem 1957, 7, 255–318. [Google Scholar]
- Ishiwata, S; Shimamoto, Y; Sasaki, D; Suzuki, M. Molecular synchronization in actomyosin motors from single molecule to muscle fiber via nanomuscle. In Sliding Filament Mechanism in Muscle Contraction; Sugi, H, Ed.; Springer: Berlin, 2005; pp. 25–36. [Google Scholar]
- Jarosch, R. The thin filament rotation model of muscle contraction. In 2nd Vienna Muscle Symposium Proceedings; Frey, M, Freilinger, G, Eds.; Facultas Universitätsverlag: Wien, 1986; pp. 1–7. [Google Scholar]
- Luther, PK; Barry, JS; Squire, JM. The three-dimensional structure of a vertebrate wide slow muscle Z-band: Lessons on Z-band assembly. J. Mol. Biol 2002, 315, 9–20. [Google Scholar]
- Akimoto, T; Sugi, H. Structural origin of the series elastic component in horseshoe crab skeletal muscle fibers. Comp. Biochem. Physiol. A 1999, 122, 139–144. [Google Scholar]
- Matsubara, I; Umazume, Y; Yagi, N. Lateral filamentary spacing in chemically skinned murine muscles during contraction. J. Physiol 1985, 360, 135–148. [Google Scholar]
- Cecchi, G; Bagni, MA; Griffiths, PJ; Ashley, CC; Maeda, Y. Dedection of radial crossbridge force by lattice spacing changes in intact single muscle fibers. Science 1990, 250, 1409–1411. [Google Scholar]
- Hanson, J; Huxley, HE. The structural basis of contraction in striated muscle. Symp. Soc. Exp. Biol 1955, 9, 228–264. [Google Scholar]
- Hanson, J; Huxley, HE. Quantitative studies in the structure of cross-striated myofibrils: II. Investigations by biochemical techniques. Biochim. Biophys. Acta 1957, 23, 250. [Google Scholar]
- Tamura, Y; Hatta, I; Matsuda, T; Sugi, H; Tsuchiya, T. Changes in muscle stiffness during contraction recorded using ultrasonic waves. Nature 1982, 299, 631–633. [Google Scholar]
- Hatta, I; Sugi, H; Tamura, Y. Stiffness changes in frog skeletal muscle during contraction recorded using ultrasonic waves. J. Physiol 1988, 403, 193–209. [Google Scholar]
- Hill, DK. Changes in transparency of muscle during a twitch. J. Physiol 1949, 108, 292–302. [Google Scholar]
- Rauh, F. Die Latenzzeit des Muskelelementes. Z. Biol 1922, 76, 25–48. [Google Scholar]
- Fischer, E. Die Zerlegung der Muskelzuckung in Teilfunktionen III. Pflügers Arch 1926, 213, 353–369. [Google Scholar]
- Schaefer, H; Göpfert, H. Aktionsstrom und optisches Verhalten des Froschmuskels in ihrer zeitlichen Beziehung zur Zuckung. Pflügers Arch 1937, 238, 684–708. [Google Scholar]
- Sandow, A. Studies on the latent period of muscular contraction. General properties of latency relaxation. J. Cell. Comp. Physiol 1944, 24, 221–256. [Google Scholar]
- Hill, AV. The earliest manifestation of the mechanical response of striated muscle. Proc. R. Soc. Lond. B. Biol. Sci 1951, 138, 339–348. [Google Scholar]
- Abbott, BC; Ritchie, JM. Early tension relaxation during a muscle twitch. J. Physiol 1951, 113, 330–335. [Google Scholar]
- Guld, C; Sten-Knudsen, O. Correlation of isometric twitch tension and latency relaxation to the sarcomere length in frog muscle fibres. Acta Physiol. Scand 1960, 50(suppl. 175), 63–65. [Google Scholar]
- Sandow, A. Latency relaxation: A brief analytical review. Med. Coll. Virginia Q 1966, 2, 82–89. [Google Scholar]
- Mulieri, LA. The dependence of the latency relaxation on sarcomere length and other characteristics of isolated muscle fibres. J. Physiol 1972, 223, 333–335. [Google Scholar]
- Hill, DK. Tension due to interaction between the sliding filaments in resting striated muscle. The effect of stimulation. J. Physiol 1968, 199, 637–684. [Google Scholar]
- Sten-Knudsen, O. Torsional elasticity of the isolated cross striated muscle fibre. Acta Physiol. Scand 1953, 28(suppl. 104), 1–240. [Google Scholar]
- Proske, U; Morgan, DL. Do cross-bridges contribute to the tension during stretch of passive muscle? J. Muscle Res. Cell Mot 1999, 20, 433–442. [Google Scholar]
- Lännergren, J. The effect of low-level activation on the mechanical properties of isolated frog muscle fibers. J. Gen. Physiol 1971, 58, 145–162. [Google Scholar]
- Moss, RL; Sollins, MR; Julian, FJ. Calcium activation produces a characteristic response to stretch in both skeletal and cardiac muscle. Nature 1976, 260, 619–621. [Google Scholar]
- Sandow, A. Skeletal muscle. Annu. Rev. Physiol 1970, 32, 87–138. [Google Scholar]
- Chalovich, JM; Chock, PE; Eisenberg, E. Mechanism of action of troponin-tropomyosin inhibition of actomyosin ATPase activity without inhibition of myosin binding to actin. J. Biol. Chem 1981, 256, 575–578. [Google Scholar]
- Tsukita, S; Yano, M. Actomyosin structure in contracting muscle detected by rapid freezing. Nature 1985, 317, 182–184. [Google Scholar]
- Schoenberg, M. Characterization of the myosin adenosine triphosphate M.ATP crossbridge in rabbit and frog muscle fibres. Biophys. J 1988, 54, 135–148. [Google Scholar]
- Brenner, B. Muscle mechanics and biochemical kinetics. In Molecular Mechanism of Muscular Contraction; Squire, J, Ed.; Macmillan Press: Southampton, 1990; pp. 47–149. [Google Scholar]
- Bozler, E. Die mechanischen Eigenschaften des ruhenden Muskels, ihre experimentelle Beeinflussung und physiologische Bedeutung. Z. vergl. Physiol 1931, 14, 429. [Google Scholar]
- Bozler, E. An analysis of the properties of smooth muscle. Cold Spring Harbor Symp. Quant. Biol 1936, 4, 260. [Google Scholar]
- Needham, DM. Machina carnis; The University Press: Cambridge, 1971. [Google Scholar]
- Blix, M. Die Länge und die Spannung des Muskels. Zweite Abhandlung. Skan. Arch. Physiol 1893, 4, 399–409. [Google Scholar]
- Bagni, MA; Cecchi, G; Colomo, F; Garzella, P. Are weakly binding bridges present in resting intact muscle fibres? Biophys. J 1992, 63, 1412–1415. [Google Scholar]
- Bagni, MA; Cecchi, G; Colombini, B; Colomo, F. A non-cross-bridge stiffness in activated frog muscle fibres. Biophys. J 2002, 82, 3118–3127. [Google Scholar]
- Bagni, MA; Colombini, B; Colomo, F; Palmini, RB; Cecchi, G. Non Cross-bridge stiffness in skeletal muscle fibres at rest and during activity. In Sliding Filament Mechanism in Muscle Contraction; Sugi, H, Ed.; Springer: Berlin, 2005; pp. 105–155. [Google Scholar]
- Cecchi, G; Griffiths, PJ; Taylor, S. Muscle contraction: Kinetics of crossbridge attachment studied by high-frequency stiffness measurements. Science 1982, 217, 70–72. [Google Scholar]
- Linari, M; Reedy, MK; Reedy, MC; Lombardi, V; Piazzesi, G. Ca-Activation and stretch-activation in insect flight muscle. Biophys. J 2004, 87, 1101–1111. [Google Scholar]
- Schwann, T. Handbuch der Physiologie des Menschen; Müller, J, Ed.; J. Hölscher: Coblenz, 1837; Vol. 2, pp. 59–62. [Google Scholar]
- Weber, E. Muskelbewegung. In Handwörterbuch der Physiologie mit Rücksicht auf physiologische Pathologie; Wagner, R, Ed.; F. Vieweg und Sohn: Braunschweig, 1846; ; III. Band, pp. 1–122. [Google Scholar]
- Fick, A. Mechanische Arbeit und Wärmeentwicklung bei der Muskelthätigkeit; Brockhaus: Leipzig, 1882. [Google Scholar]
- Heidenhain, R. Mechanische Leistung, Wärmeentwicklung und Stoffumsatz bei der Muskelthätigkeit; Breitkopf und Härtel: Leipzig, 1864. [Google Scholar]
- Morgan, DL; Prochazka, A; Proske, U. The after-effects of stretch and fusimotor stimulation on the responses of primary endings of cat muscle spindles. J. Physiol 1984, 356, 465–477. [Google Scholar]
- Campbell, KS; Lakie, M. A cross-bridge mechanism can explain the thixotropic short-range elastic component of relaxed frog skeletal muscle. J. Physiol 1998, 510, 941–962. [Google Scholar]
- Hill, AV. The abrupt transition from rest to activity in muscle. Proc. R. Soc. Lond. B. Biol. Sci 1949, 136, 399–420. [Google Scholar]
- Yasuda, R; Miyata, H; Kinosita, K, Jr. Direct measurement of the torsional rigidity of single actin filaments. J. Mol. Biol 1996, 263, 227–236. [Google Scholar]
- Davies, RE; Kushmerick, MJ; Larson, RE. ATP, activation and the heat of shortening of muscle. Nature 1976, 214, 148. [Google Scholar]
- Ebashi, S; Kodama, A; Ebashi, F. Troponin. 1. Preparation and physiological function. J. Biochem 1968, 64, 465–477. [Google Scholar]
- Huxley, HE; Brown, W. The low-angle X-ray diagram of vertebrate striated muscle and its behaviour during contraction and rigor. J. Mol. Biol 1967, 30, 383–434. [Google Scholar]
- Huxley, HE. Structural difference between resting and rigor muscle; evidence from intensity changes in the low-angle equatorial X-ray diagram. J. Mol. Biol 1968, 37, 507. [Google Scholar]
- Zhao, F-Q; Craig, R. Ca2+ causes release of myosin heads from the thick filament surface on the milliseconds time scale. J. Mol. Biol 2003, 327, 145–158. [Google Scholar]
- Bailey, K. Tropomyosin: A new asymmetric protein component of the muscle fibril. J. Biochem 1948, 43, 271–278. [Google Scholar]
- Cummins, P; Perry, SV. The subunits and biological activity of polymorphic forms of tropomyosin. Biochem. J 1972, 128, 106P–107P. [Google Scholar]
- Stone, D; Sodek, J; Johnson, P; Smillie, LB. Proc. IX FEBS Meeting Budapest 1975, 31, 125–136.
- Crick, FHC. Is α-keratin a coiled coil? Nature 1952, 170, 882–883. [Google Scholar]
- Crick, FHC. The packing of α-helices: simple coiled-coils. Acta Cryst 1953, 6, 689–697. [Google Scholar]
- Blout, ER; Idelson, M. Compositional effects on the configuration of water-soluble polypeptide copolymers of L-Glutamic acid and L-Lysin. J. Amer. Chem. Soc 1958, 80, 4909–4913. [Google Scholar]
- Cohen, C; Holmes, KC. X-Ray diffraction evidence for α-helical coiled-coils in native muscle. J. Mol. Biol 1963, 6, 423–432. [Google Scholar]
- Pauling, L; Corey, RB. Compound helical configurations of polypeptide chains: Structure of proteins of the alpha-keratin type. Nature 1953, 171, 59–62. [Google Scholar]
- Corey, RB; Pauling, L. The configuration of polypeptide chains in proteins. Inst. Lombardo Rend. Sci 1955, 89, 10–37. [Google Scholar]
- Bailey, K. Structure proteins II. Muscle. In The Proteins; Neurath, H, Bailey, K, Eds.; Academic Press: New York, 1954; p. 951. [Google Scholar]
- Morales, MF. Mechanisms of muscle contraction. Rev. Mod. Phys 1959, 31, 426. [Google Scholar]
- Kuntz, ID; Grippen, GM; Collman, PA; Kimelman, D. Calculation of protein tertiary structure. J. Mol. Biol 1976, 106, 983–994. [Google Scholar]
- Gaffin, RD; Gokulan, K; Sacchettini, JC; Hewett, T; Klevitsky, R; Robbins, J; Muthuchamy, M. Charged residue changes in the carboxyl terminus of α-tropomyosin alter mouse cardiac muscle contractility. J. Physiol 2004, 556, 531–543. [Google Scholar]
- Jarosch, R. The cross-bridge cycle as a passive process. J. Muscle Res. Cell Mot 1980, 1, 448–449. [Google Scholar]
- Jarosch, R. Das sterische Verhalten der Alpha-Helix. Z. Naturforsch 1969, 24B, 672–680. [Google Scholar]
- Fraser, RDB; Macrae, TP. Conformation in fibrous proteins; Academic Press: London, 1973. [Google Scholar]
- Cohen, C. The protein switch of muscle contraction. Sci. Am 1975, 233, 36–45. [Google Scholar]
- Flicker, PF; Phillips, GN, Jr; Cohen, C. Structure of troponin and its interaction with tropomyosin. J. Mol. Biol 1984, 162, 495–501. [Google Scholar]
- White, SP; Cohen, C; Phillips, GN, Jr. Structure of co-crystals of tropomyosin and troponin. Nature 1987, 325, 826–828. [Google Scholar]
- Ohtsuki, I. Molecular basis of calcium regulation of striated muscle contraction. In Sliding Filament Mechanism in Muscle Contraction; Sugi, H, Ed.; Springer: Berlin, 2005; pp. 223–231. [Google Scholar]
- Brown, JH; Kim, KH; Jun, G; Greenfield, NJ; Dominguez, R; Volkman, N; Hitchcock DeGregori, SE; Cohen, C. Deciphering the design of the tropomyosin molecule. Proc. Natl. Acad. Sci. USA 2001, 98, 8496–8501. [Google Scholar]
- Haselgrove, J. X-ray evidence for a conformational change in actin-containing filaments of vertebrate striated muscle. Cold Spring Harbor Symp. Quant. Biol 1972, 37, 341–352. [Google Scholar]
- Huxley, HE. Structural changes in actin- and myosin-containing filaments during contraction. Cold Spring Harb. Symp. Quant. Biol 1972, 37, 361–376. [Google Scholar]
- Parry, DAD; Squire, JM. Structural role of tropomyosin in muscle regulation: Analysis of the X-ray diffraction patterns from relaxed and contracting muscles. J. Mol. Biol 1973, 75, 33–55. [Google Scholar]
- Lehman, WR; Craig, R; Vibert, P. Ca2+ induced tropomyosin movement in Limulus thin filaments revealed by three-dimensional reconstruction. Nature 1994, 368, 65–67. [Google Scholar]
- Reuben, JP; Brandt, PW; Berman, M; Grundfest, H. Regulation of tension in the skinned crayfish muscle fiber. I. Contraction and relaxation in the absence of Ca (pCa is greater than 9). J. Gen. Physiol 1971, 57, 385–407. [Google Scholar]
- Brandt, PW; Schachat, TH. Troponin C modulates the activation of thin filaments by rigor cross-bridges. Biophys. J 1997, 72, 2262–2267. [Google Scholar]
- Orlova, A; Egelman, H. F-actin retains a memory of angular order. Biophys. J 2000, 78, 2180–2185. [Google Scholar]
- Kress, M; Huxley, HE; Faruqui, AR; Hendrix, J. Structural changes during activation of frog muscle studied by time-resolved X-ray diffraction. J. Mol. Biol 1986, 188, 325–342. [Google Scholar]
- Harford, JJ; Squire, JM. Evidence for structurally different attached states of myosin cross-bridges on actin during contraction of fish muscle. Biophys. J 1992, 63, 387–396. [Google Scholar]
- Squire, JM; Knupp, C; Roessle, M; Al-Khayat, HA; Irving, TC; Eakins, F; Mok, N-S; Harford, JJ; Reedy, MK. X-ray diffraction studies of striated muscles. In Sliding Filament Mechanism in Muscle Contraction; Sugi, H, Ed.; Springer: Berlin, 2005; pp. 45–60. [Google Scholar]
- Squire, JM; Morris, EP. A new look at thin filament regulation in vertebrate skeletal muscle. FASEB J 1998, 12, 761–771. [Google Scholar]
- Weber, A; Hasselbach, W. Die Erhöhung der Rate der ATP-Spaltung durch Myosin- und Actomyosin-Gele bei Beginn der Spaltung. Biochim. Biophys. Acta 1954, 15, 237. [Google Scholar]
- Cecchi, G; Colomo, F; Lombardi, V. Force-velocity relation in normal and nitrate-treated frog single muscle fibres during rise of tension in an isometric tetanus. J. Physiol 1978, 285, 257–273. [Google Scholar]
- He, Z-H; Chillingworth Brune, M; Corrie, JET; Trentham, DR; Webb, MR; Ferenczi, MA. ATPase kinetics on activation of rabbit and frog permeabilized isometric muscle fibres: A real time phosphate assay. J. Mol. Biol 1997, 216, 49–68. [Google Scholar]
- Josephson, RK; Edman, KA. Changes in the maximum speed of shortening of frog muscle fibres in a tetanic contraction and during relaxation. J. Physiol 1998, 507, 511–525. [Google Scholar]
- Harrington, WF. A mechanochemical mechanism for muscle contraction. Proc. Natl. Acad. Sci. USA 1971, 68, 685–689. [Google Scholar]
- Harrington, WF. On the origin of the contractile force in skeletal muscle. Proc. Natl. Acad. Sci. USA 1979, 76, 5066–5070. [Google Scholar]
- Harrington, WF; Ueno, H; Davis, JS. Helix-coil melting in rigor and activated cross-bridges of skeletal muscle. In Molecular Mechanics of Muscle Contraction; Sugi, H, Pollack, GH, Eds.; Plenum: New York, 1988; pp. 307–318. [Google Scholar]
- Harrington, WF; Karr, T; Busa, WB; Lowell, SJ. Contraction in myofibrils in the presence of antibodies to myosin subfragment 2. Proc. Natl. Acad. Sci. USA 1990, 87, 7453–7456. [Google Scholar]
- Lovell, S; Karr, T; Harrington, WF. Suppression of contractile force in muscle fibers by antibody to myosin subfragment 2. Proc. Natl. Acad. Sci. USA 1988, 85, 1849–1853. [Google Scholar]
- Sugi, H; Kobayashi, T; Gross, T; Noguchi, K; Karr, T; Harrington, WF. Contraction characteristics and ATPase activity of skeletal muscle fibers in the presence of antibody to myosin subfragment 2. Proc. Natl. Acad. Sci. USA 1992, 89, 6134–6137. [Google Scholar]
- Tsuchiya, T; Tanaka, H; Shirakawa, I; Karr, T; Sugi, H. Evidence for the essential role of myosin subfragment-2 in the ATP-dependent actin-myosin sliding in muscle contraction. Japan. J. Physiol 1998, 48, 383–387. [Google Scholar]
- Blanchard, A; Ohanian, V; Critchley, D. The structure and function of α-actinin. J. Muscle Res. Cell Mot 1989, 10, 280–289. [Google Scholar]
- Tang, J; Taylor, DW; Taylor, KA. The three-dimensional structure of alpha-actinin obtained by cryoelectron microscopy suggests a model for Ca2+-dependent actin binding. J. Mol. Biol 2001, 310, 845–858. [Google Scholar]
- Liu, J; Taylor, DW; Taylor, KA. A 3-D Reconstruction of smooth muscle α-actinin by cryo EM reveals two different conformations at the actin-binding region. J. Mol. Biol 2004, 338, 115–125. [Google Scholar]
- Endo, M. Length dependence of activation of skinned muscle fibres by calcium. Cold Spring Harbor Symp. Quant. Biol 1973, 37, 505–510. [Google Scholar]
- Stephenson, OG; Wendt, IR. Length dependence of changes in sarcoplasmic calcium concentration and myofibrillar calcium sensitivity. J. Muscle Res. Cell Mot 1984, 5, 243–272. [Google Scholar]
- Balnave, CD; Allen, DG. The effect of muscle length on intracellular calcium and force in single fibres from mouse skeletal muscle. J. Physiol. (Lond.) 1996, 492, 705–713. [Google Scholar]
- Ridgway, EB; Gordon, AM. Muscle calcium transient: effect of post-stimulus length changes in single muscle fibers. J. Gen. Physiol 1984, 83, 75–103. [Google Scholar]
- Gordon, AM; Ridgway, EB. Stretch of active muscle during the declining phase of the calcium transient produces biphasic changes in calcium binding to the activating sites. J. Gen. Physiol 1990, 96, 1013–1035. [Google Scholar]
- Kurihara, S; Komukai, K. Tension-dependent changes of intracellular Ca2+ transients in ferret ventricular muscles. J. Physiol 1995, 489, 617–625. [Google Scholar]
- Fenn, WO. A quantitative comparison between the energy liberated and the work performed by the isolated sartorius muscle of the frog. J. Physiol 1923, 58, 175–203. [Google Scholar]
- Fenn, WO. The relation between the work performed and the energy liberated in muscular contraction. J. Physiol 1924, 58, 373–395. [Google Scholar]
- Hill, AV. The effect of load on the heat of shortening of muscle. Proc. R. Soc. Lond. B. Biol. Sci 1964, 159, 297–318. [Google Scholar]
- Curtin, NA; Woledge, RC. Effect of muscle length on energy balance in frog skeletal muscle. J. Physiol. (Lond.) 1981, 316, 453–468. [Google Scholar]
- Julian, FJ; Sollins, KR; Sollins, MR. Regulation of force and speed of shortening in muscle contraction. Cold Spring Harbor Symp. Quant. Biol 1973, 37, 635–646. [Google Scholar]
- Edman, KAP; Mulieri, LA; Scubon-Mulieri, B. Non-hyperbolic force-velocity relationship in single muscle fibres. Acta Physiol. Scand 1976, 98, 143–156. [Google Scholar]
- Lännergren, J. The force-velocity relation of isolated twitch and slow muscle fibres of Xenopus laevis. J. Physiol 1978, 283, 501–521. [Google Scholar]
- Lännergren, J; Lindblom, P; Johansson, B. Contractile properties of two varieties of twitch fibres in Xenopus laevis. Acta Physiol. Scand 1982, 114, 523–535. [Google Scholar]
- Edman, KAP. Double-hyperbolic force-velocity relation in frog muscle fibres. J. Physiol 1988, 404, 301–321. [Google Scholar]
- Edman, KAP. Mechanism underlying double-hyperbolic force-velocity relation in vertebrate skeletal muscle. In Mechanism of Myofilament Sliding in Muscle Contraction; Sugi, H, Pollack, GH, Eds.; Plenum: New York, 1993; pp. 667–678. [Google Scholar]
- Huxley, AF; Peachey, LD. The maximum length of contraction in vertebrate striated muscle. J. Physiol 1961, 156, 150–165. [Google Scholar]
- Gordon, AM; Huxley, AF; Julian, FJ. The variation of isometric tension with sarcomere length in vertebrate muscle fibres. J. Physiol 1966, 184, 170–192. [Google Scholar]
- Stephenson, DG; Williams, DA. Effects of sarcomere length on the force-pCa relation in fast- and slow-twitch skinned muscle fibres from rat. J. Physiol 1982, 333, 637–653. [Google Scholar]
- Edman, KAP. The velocity of unloaded shortening and its relation to sarcomere length and isometric force in vertebrate muscle fibres. J. Physiol 1979, 291, 143–159. [Google Scholar]
- Sugi, H; Ohta, T; Tameyasu, T. Development of the maximum isometric force at short sarcomere length in calcium-activated muscle myofibrils. Experientia 1983, 39, 147–148. [Google Scholar]
- Huxley, AF; Simmons, RM. Mechanical transients and the origin of muscular force. Cold Spring Harb. Symp. Quant. Biol 1973, 37, 669–680. [Google Scholar]
- Jarosch, R; Foissner, I. A rotation model for microtubule and filament sliding. Eur. J. Cell Biol 1982, 26, 295–302. [Google Scholar]
- Bugnard, L. The relation between total and initial heat in single muscle twitches. J. Physiol 1934, 82, 509–519. [Google Scholar]
- Gilbert, C; Kretzschmar, KM; Wilkie, DR; Woledge, R. Chemical change and energy output during muscular contraction. J. Physiol 1971, 218, 163–193. [Google Scholar]
- Homsher, E; Kean, CJ; Wallner, A; Garibian-Sarian, V. The time course of energy balance in an isometric tetanus. J. Gen. Physiol 1979, 73, 553–567. [Google Scholar]
- Paul, RJ. Physical and biochemical energy balance during an isometric tetanus and steady state recovery in frog sartorius at 0 °C. J. Gen. Physiol 1983, 81, 337–354. [Google Scholar]
- Shimamoto, Y; Kono, F; Suzuki, M; Ishiwata, S. Non-linear force-length relationship in the ADP-induced contraction of skeletal myofibrils. Biophys. J 2007, 93, 4330–4341. [Google Scholar]
- Shimamoto, Y; Suzuki, M; Ishiwata, S. Length-dependent activation and auto-oscillation in skeletal myofibrils at partial activation by Ca2+. Biochem. Biophys. Res. Commun 2008, 366, 233–238. [Google Scholar]
- Hill, AV. Chemical change and mechanical response in stimulated muscle. Proc. R. Soc. Lond. B. Biol. Sci 1953, 141, 314–320. [Google Scholar]
- Homsher, E. Muscle enthalpy production and its relationship to actomyosin ATPase. Annu. Rev. Physiol 1987, 49, 673–690. [Google Scholar]
- Hill, AV; Hartree, W. The thermo-elastic properties of muscle. Philos. Trans. Royal Soc. B 1920, 210, 153–173. [Google Scholar]
- Hill, AV. The maximum work and the mechanical efficiency of human muscles, and the most economical speed. J. Physiol 1922, 56, 19–41. [Google Scholar]
- Lupton, H. An analysis of the effects of speed on the mechanical efficiency of human muscular movement. J. Physiol 1923, 57, 337–353. [Google Scholar]
- Wyman, J, Jr. Studies on the relation of work and heat in tortoise muscle. J. Physiol 1926, 61, 337–352. [Google Scholar]
- Holroyd, SM; Gibbs, GG. The energetics of shortening amphibian cardiac muscle. Am. J. Physiol 1993, 424, H200–208. [Google Scholar]
- Matsubara, I; Yagi, N; Endoh, M. Movement of myosin heads during a heart beat. Nature 1979, 278, 474–476. [Google Scholar]
- Suga, H. Mysterious beauty of beating heart: Cardiac mechano-energetico-informatics. In Sliding Filament Mechanism in Muscle Contraction; Sugi, H, Ed.; Springer: Berlin, 2005; pp. 303–317. [Google Scholar]
- Pollack, GH; Iwazumi, T; ter Keurs, HEDJ; Shibata, EF. Sarcomere shortening in striated muscle occurs in stepwise fashion. Nature 1977, 268, 757–759. [Google Scholar]
- Pollack, GH; Vassallo, DV; Jacobson, RC; Iwazumi, T; Delay, MJ. Discrete nature of sarcomere shortening in striated muscle. In Cross-bridge Mechanism in Muscle Contraction; Sugi, H, Pollack, GH, Eds.; Univ. of Tokyo Press: Tokyo, 1979; pp. 23–24. [Google Scholar]
- Pollack, GH; Blyakhman, FA; Liu, X; Nagornyak, E. Sarcomere dynamics, stepwise shortening and the nature of contraction. In Sliding Filament Mechanism in Muscle Contraction; Sugi, H, Ed.; Springer: Berlin, 2005; pp. 113–126. [Google Scholar]
- Yakovenko, O; Blyakhman, F; Pollack, GH. Fundamental step size in single cardiac and skeletal sarcomeres. Am. J. Physiol. (Cell) 2002, 283, C735–C743. [Google Scholar]
- Liu, X; Pollack, GH. Stepwise sliding of single actin and myosin filaments. Biophys. J 2004, 86, 353–358. [Google Scholar]
- Hill, AV. First and Last Experiments in Muscle Mechanics; Cambridge University Press, 1970. [Google Scholar]
- Eastwood, AB; Wood, DS; Reuben, JB. Unusual thick and thin filament packing in a crustacean muscle. J. Cell Biol 1978, 77, 48–58. [Google Scholar]
- Gasser, HS; Hill, AV. The dynamics of muscular contraction. Proc. Roy. Soc 1924, B96, 398–437. [Google Scholar]
- Galler, S; Hilber, K. Unloaded shortening of skinned mammalian skeletal muscle fibres: Effects of the experimental approach and passive force. J. Muscle Res. Cell Mot 1994, 15, 400–412. [Google Scholar]
- Galler, S; Schmitt, TL; Pette, D. Stretch activation, unloaded shortening velocity, and myosin heavy chain isoforms of rat skeletal muscle fibres. J. Physiol 1994, 478, 513–521. [Google Scholar]
- Ford, LE; Huxley, AF; Simmons, RM. Mechanism of early tension recovery after quick release in tetanized muscle fibres. J. Physiol 1974, 240, 42–43P. [Google Scholar]
- Jewell, BR; Wilkie, DR. An analysis of the mechanical components in frog’s striated muscle. J. Physiol 1958, 143, 515–540. [Google Scholar]
- Ford, LE; Huxley, AF; Simmons, RM. Tension responses to sudden length changes in stimulated frog muscle fibres near slack length. J. Physiol 1977, 269, 441–515. [Google Scholar]
- Lombardi, V; Piazzesi, G; Linari, M. Rapid regeneration of the actin-myosin power stroke in contracting muscle. Nature 1992, 355, 638–641. [Google Scholar]
- Irving, M; Lombardi, V; Piazzesi, G; Ferenczi, MA. Myosin head movements are synchronous with the elementary force-generating process in muscle. Nature 1992, 357, 156–158. [Google Scholar]
- Hill, AV. The effect of tension in prolonging the active state in a twitch. Proc. R. Soc. Lond. B. Biol. Sci 1964, 159, 589–595. [Google Scholar]
- Blangé, T; Klaremaker, JM; Kramer, AEJL. Elasticity as an expression of cross-bridge activity in rat muscle. Pflügers Arch 1972, 336, 277–288. [Google Scholar]
- Huxley, AF; Simmons, RM. Mechanical properties of the cross-bridges of frog striated muscle. J. Physiol 1971, 218, 59–60P. [Google Scholar]
- Hilber, K; Galler, S. Mechanical properties and myosin heavy chain isoform composition of skinned skeletal muscle fibres from a human biopsy sample. Pflügers Arch 1997, 434, 551–558. [Google Scholar]
- Galler, S; Hilber, K; Pette, D. Stretch activation and myosin heavy chain isoforms of rat, rabbit and human skeletal muscle fibres. J. Muscle Res. Cell Mot 1997, 18, 441–448. [Google Scholar]
- Galler, S; Hilber, K; Pette, D. Force responses following stepwise length changes of rat skeletal muscle fibre types. J. Physiol 1996, 493, 219–227. [Google Scholar]
- Blinks, JR; Rudel, R; Taylor, SR. Calcium transients in isolated amphibian skeletal muscle fibres: Detection with aequorin. J. Physiol 1978, 277, 291–323. [Google Scholar]
- Lombardi, V; Piazzesi, G. Contraction kinetics studied by stretching single fibres from frog skeletal muscle. J. Muscle Res. Cell Mot 1990, 11, 73–74. [Google Scholar]
- Ferenczi, MA; Goldman, YE; Simmons, RM. The dependence of force and shortening velocity on substrate concentration and skinned fibres from frog muscle. J. Physiol 1984, 350, 519–543. [Google Scholar]
- Kuhn, HJ; Kulik, R; Winkler, H; Pferrer, S. Mechanochemistry of ATP binding to myosin heads in skinned Limulus fibres and skinned heart muscle fibres. J. Muscle Res. Cell Mot 1985, 6, 106. [Google Scholar]
- Anderl, R; Kuhn, HJ. The effect of MgATP on the force transients following quick length changes in skinned Limulus telson levator fibres. J. Muscle Res. Cell. Mot 1990, 11, 69–70. [Google Scholar]
- Wilkie, DR. Measurement of the series elastic component at various times during a single muscle twitch. J. Physiol 1956, 134, 527–530. [Google Scholar]
- Podolsky, RJ. Kinetics of muscular contraction: the approach to the steady state. Nature 1960, 188, 666–668. [Google Scholar]
- Armstrong, CM; Huxley, AF; Julian, FJ. Oscillatory responses in frog skeletal muscle fibres. J. Physiol 1966, 186, 26–27P. [Google Scholar]
- Huxley, AF. Muscular contraction. J. Physiol 1974, 243, 1–43. [Google Scholar]
- Sugi, H; Tsuchiya, T. Isotonic velocity transients in frog muscle fibres following quick changes in load. J. Physiol 1981, 319, 219–238. [Google Scholar]
- Civan, MM; Podolsky, RJ. Contraction kinetics of striated muscle fibres following quick changes in load. J. Physiol 1966, 184, 511–534. [Google Scholar]
- Galler, S. Stretch activation of skeletal muscle fibre types. Pflügers Arch 1994, 427, 384–386. [Google Scholar]
- Brenner, B. Muscle mechanics II: Skinned muscle fibres. In Current Methods in Muscle Physiology; Sugi, H, Ed.; Oxford University Press, 1998; pp. 33–69. [Google Scholar]
- Herzog, W; Leonard, TR; Wu, JZ. Force depression following skeletal muscle shortening is long lasting. J. Biomechanics 1998, 31, 1163–1168. [Google Scholar]
- Herzog, W; Lee, EJ; Rassier, DE. Residual force enhancement in skeletal muscle. J. Physiol 2006, 574, 635–642. [Google Scholar]
- Edman, KAP; Elzinga, G; Noble, MIM. Stretch of contracting muscle fibers: Evidence for regularly spaced sites along the filaments and enhanced mechanical performance. In Contractile Mechanism in Muscle; Pollack, GH, Sugi, H, Eds.; Plenum Press: New York, 1984; pp. 737–751. [Google Scholar]
- Abbott, BC; Aubert, XM. The force exerted by active striated muscle during and after change of length. J. Physiol 1952, 117, 77–86. [Google Scholar]
- Griffiths, PJ; Güth, K; Kuhn, HJ; Ruegg, JC. Cross-bridge slippage in skinned frog muscle fibres. Biophys. Struct. Mech 1980, 7, 107–124. [Google Scholar]
- Seemann, J. Über den Einfluß der Belastung auf den Kontraktionsakt. I. Wirkung von Spannungsänderungen auf die isometrische Zuckung. Pflügers Arch 1905, 106, 420–458. [Google Scholar]
- Pollack, GH; Horowitz, A. Length-tension relations in slightly pre-shortened sarcomeres. J. Muscle Res. Cell Mot 1990, 11, 69. [Google Scholar]
- Sugi, H; Tsuchiya, T. Stiffness changes during enhancement and deficit of isometric force by slow length changes in frog skeletal muscle fibres. J. Physiol 1988, 407, 215–229. [Google Scholar]
- Julian, FJ; Sollins, MR. Variation of muscle stiffness with force at increasing speeds of shortening. J. Gen. Physiol 1975, 66, 287–302. [Google Scholar]




















© 2008 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/). This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Jarosch, R. Large-scale Models Reveal the Two-component Mechanics of Striated Muscle. Int. J. Mol. Sci. 2008, 9, 2658-2723. https://doi.org/10.3390/ijms9122658
Jarosch R. Large-scale Models Reveal the Two-component Mechanics of Striated Muscle. International Journal of Molecular Sciences. 2008; 9(12):2658-2723. https://doi.org/10.3390/ijms9122658
Chicago/Turabian StyleJarosch, Robert. 2008. "Large-scale Models Reveal the Two-component Mechanics of Striated Muscle" International Journal of Molecular Sciences 9, no. 12: 2658-2723. https://doi.org/10.3390/ijms9122658
APA StyleJarosch, R. (2008). Large-scale Models Reveal the Two-component Mechanics of Striated Muscle. International Journal of Molecular Sciences, 9(12), 2658-2723. https://doi.org/10.3390/ijms9122658



