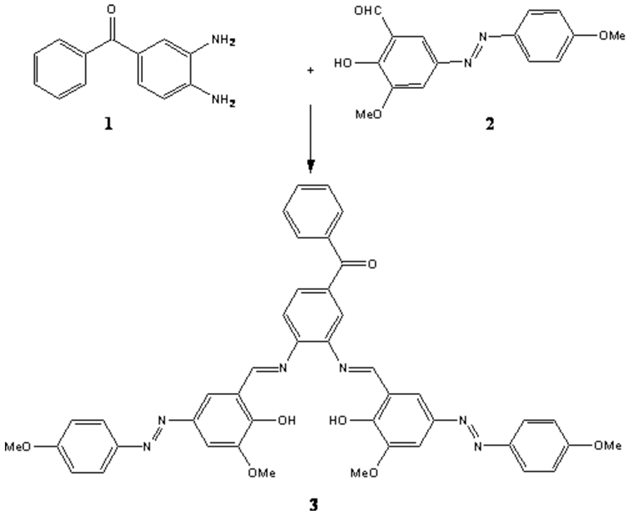
Synthesis of (3,4-bis{[2-hydroxy-3-methoxy-5-(4-methylphenyl azo)benzylidene]-amino}phenyl) phenyl methanone as a novel azo Schiff base
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgment
References
- Aydogan, F.; Öcal, N.; Turgut, Z.; and Yolacan, C. Bull. Korean Chem.Soc. 2001, 22, 476–480.
- Kabeer, A . S.; Baseer, M. A.; Mote, N. A. Asian J. Chem. 2001, 13(2), 496–500.
- El-masry, A. H.; Fahmy, H. H.; Abdelwahed, S. H. A. Molecules 2000, 5, 1429–1438. [CrossRef]
- More, P. G.; Bhalvankar, R. B.; Pattar, S. C. J. Indian Chem. Soc. 2001, 78(9), 474–475.
- Pandeya, S. N.; Sriram, D.; Nath, G.; De Clercq, E. IL Farmaco 1999, 54, 624–628.
- Singh, W.M.; Dash, B.C. Pesticides 1988, 22(11), 33–37.
- Hodnett, E. M.; Mooney, P. D. J. Med. Chem. 1970, 13, 786. [CrossRef] [PubMed]
- Hodnett, E. M.; Dunn, W. J. J. Med. Chem. 1970, 13, 768–770. [CrossRef] [PubMed]
- Nofal, Z. M. N.; El-Zahar, M. I.; Abd El-Karim, S. S. Molecules 2000, 5, 99–113. [CrossRef]
- Samadhiya, S.; Halve, A. Orient. J. Chem. 2001, 17(1), 119–122.
- Tai, X.; Yin, X.; Chen, Q.; Tan, M. Molecules 2003, 8, 439–443. [CrossRef]
- Zollinger, H. Color Chemistry, second ed.; VCH: Weinheim, 1991. [Google Scholar]
- Croot, P. L.; Johansson, M. Electroanalysis 2000, 12, 565–576. [CrossRef]
- Choi, D.; Lee, S. K.; Chung, T. D.; Kim, H. Electroanalysis 2000, 12, 477–482. [CrossRef]
- Jolly, V. S.; Pathak, P.; Jain, R. J. Indian Chem. Soc. 1993, 70, 505–507.
- Halve, A.; Goyal, A. Orient. J. Chem. 1996, 12(1), 87–88.
- Furniss, B. S.; Hannaferd, A. J.; Rogers, V.; Smith, P. W. G.; Tatchell, A. R. Vogel’s Textbook of Practical Organic Chemistry, 4th ed.; Longman Inc.: New York, 1981; p. 716. [Google Scholar]
- Matsui, S.; Hashimoto, Y.; Saigo, K. Synthesis 1998, 1161–1166.
- Sample Availability : Available from MDPI.
© 2004 MDPI. All rights reserved.
Share and Cite
Jarrahpour, A.A.; Zarei, M. Synthesis of (3,4-bis{[2-hydroxy-3-methoxy-5-(4-methylphenyl azo)benzylidene]-amino}phenyl) phenyl methanone as a novel azo Schiff base. Molbank 2004, 2004, M376. https://doi.org/10.3390/M376
Jarrahpour AA, Zarei M. Synthesis of (3,4-bis{[2-hydroxy-3-methoxy-5-(4-methylphenyl azo)benzylidene]-amino}phenyl) phenyl methanone as a novel azo Schiff base. Molbank. 2004; 2004(1):M376. https://doi.org/10.3390/M376
Chicago/Turabian StyleJarrahpour, A. A., and M. Zarei. 2004. "Synthesis of (3,4-bis{[2-hydroxy-3-methoxy-5-(4-methylphenyl azo)benzylidene]-amino}phenyl) phenyl methanone as a novel azo Schiff base" Molbank 2004, no. 1: M376. https://doi.org/10.3390/M376
APA StyleJarrahpour, A. A., & Zarei, M. (2004). Synthesis of (3,4-bis{[2-hydroxy-3-methoxy-5-(4-methylphenyl azo)benzylidene]-amino}phenyl) phenyl methanone as a novel azo Schiff base. Molbank, 2004(1), M376. https://doi.org/10.3390/M376



