-
![<em>bis</em>(2-Phenylpyridinato)-[4,4′-<em>bis</em>(iodoethynyl)-2,2′-bipyridine]-iridium(III) Hexafluorophosphate](https://pub.mdpi-res.com/title_story/title_story_17575637166051.jpg?1760951576) bis(2-Phenylpyridinato)-[4,4′-bis(iodoethynyl)-2,2′-bipyridine]-iridium(III) Hexafluorophosphate
bis(2-Phenylpyridinato)-[4,4′-bis(iodoethynyl)-2,2′-bipyridine]-iridium(III) Hexafluorophosphate -
![Benzyl-<em>N</em>-[4-(2-hydroxyethyl)-1,3-thiazol-2-yl]carbamate](https://pub.mdpi-res.com/title_story/title_story_17574899376081.jpg?1760951576) Benzyl-N-[4-(2-hydroxyethyl)-1,3-thiazol-2-yl]carbamate
Benzyl-N-[4-(2-hydroxyethyl)-1,3-thiazol-2-yl]carbamate -
 1,3,4,5-Tetra-O-benzoyl-α-d-tagatopyranose
1,3,4,5-Tetra-O-benzoyl-α-d-tagatopyranose -
 3-Methyl-2-((methylthio)methyl)but-2-enal
3-Methyl-2-((methylthio)methyl)but-2-enal -
 (Z)-2-(Bromomethyl)-3-(hydroxymethylene)-7-methoxy-5-methyl-2-(tribromomethyl)-4-chromanone
(Z)-2-(Bromomethyl)-3-(hydroxymethylene)-7-methoxy-5-methyl-2-(tribromomethyl)-4-chromanone
Journal Description
Molbank
Molbank
is an international, peer-reviewed, open access journal comprised of a unique collection of one-compound-per-paper short notes on synthetic compounds and natural products published quarterly online by MDPI.
- Open Access— free for readers, with article processing charges (APC) paid by authors or their institutions.
- High Visibility: indexed within Scopus, ESCI (Web of Science), Reaxys, CAPlus / SciFinder, and other databases.
- Rapid Publication: manuscripts are peer-reviewed and a first decision is provided to authors approximately 14.7 days after submission; acceptance to publication is undertaken in 2.8 days (median values for papers published in this journal in the first half of 2025).
- Recognition of Reviewers: reviewers who provide timely, thorough peer-review reports receive vouchers entitling them to a discount on the APC of their next publication in any MDPI journal, in appreciation of the work done.
Impact Factor:
0.4 (2024)
Latest Articles
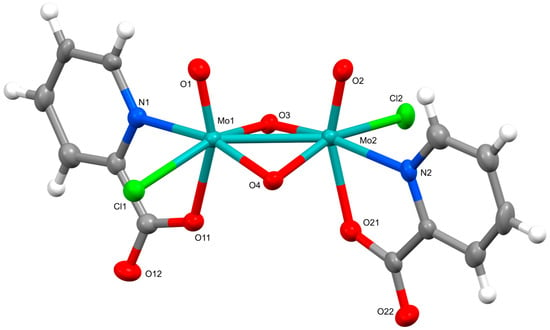
Revisiting the Coordination Chemistry of Molybdenum(V): Novel Complexes with Pyrazinoate and Picolinate Ligands
Molbank 2025, 2025(4), M2079; https://doi.org/10.3390/M2079 (registering DOI) - 24 Oct 2025
Abstract
Reactions of (pyH)5[MoOCl4(H2O)]3Cl2 with picolinic and pyrazinoic acids yielded three new dinuclear molybdenum(V) complexes: (pyH)2[Mo2O4Cl2(pic)2]·CH3CN (1), (pyH)2[Mo2
[...] Read more.
Reactions of (pyH)5[MoOCl4(H2O)]3Cl2 with picolinic and pyrazinoic acids yielded three new dinuclear molybdenum(V) complexes: (pyH)2[Mo2O4Cl2(pic)2]·CH3CN (1), (pyH)2[Mo2O4Cl2(pic)2]·CH3CH2CN (2) and (pyH)2[Mo2O4Cl2(pyraz)2]·CH3CN (3) (pic− = picolinate, pyraz− = pyrazinoate and pyH+ = protonated pyridine). The compounds were characterized by single-crystal X-ray diffraction, infrared and 1H NMR spectroscopy, elemental analysis, and TG/DSC measurements. All display a robust {MoV2O4}2+ core with the heteroaromatic ligands bound in a N,O-bidentate chelating manner.
Full article
(This article belongs to the Section Structure Determination)
►
Show Figures
Open AccessCommunication
Missing Crystal Structure and DFT Study of Calcium Complex Based on 4-(3-Hydroxy-2-methyl-4-oxopyridin-1(4H)-yl) Acetic Acid
by
Roman V. Rumyantcev, Marina A. Katkova, Galina S. Zabrodina, Georgy K. Fukin and Sergey Yu. Ketkov
Molbank 2025, 2025(4), M2080; https://doi.org/10.3390/M2080 (registering DOI) - 24 Oct 2025
Abstract
►▼
Show Figures
Recently, 3-hydroxy-4-pyridinones have been extensively studied as chelating bidentate agents of metal ions for various biomedical applications. This study reports the structural characterization and density functional theory (DFT) analysis of centrosymmetric calcium complex based on 4-(3-hydroxy-2-methyl-4-oxopyridin-1(4H)-yl) acetic acid (1).
[...] Read more.
Recently, 3-hydroxy-4-pyridinones have been extensively studied as chelating bidentate agents of metal ions for various biomedical applications. This study reports the structural characterization and density functional theory (DFT) analysis of centrosymmetric calcium complex based on 4-(3-hydroxy-2-methyl-4-oxopyridin-1(4H)-yl) acetic acid (1). The structure of complex 1 was determined by X-ray crystallography. The 3-hydroxy-4-pyridinone ligand in the studied complex is bound to the calcium ion in the desired monodentate, non-bridging manner. The calcium ion has a coordination number of six and adopts a distorted octahedral geometry. Analyzed geometric characteristics corresponding to hydrogen bonds in the crystal. The theoretical study of intra- and intermolecular interactions utilized DFT with the PBE0-D3/def2-TZVP (Gaussian Inc., Wallingford, CT, USA) level of theory. The charge redistribution in the ligand was studied in comparison with the free acid molecule.
Full article
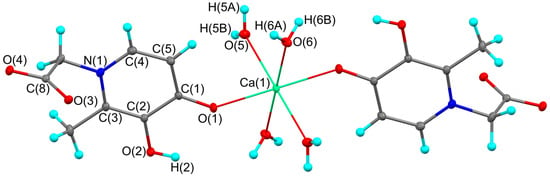
Figure 1
Open AccessShort Note
N-(2-Fluoro-2-propen-1-yl)-5-(trifluoromethyl)-2-pyridinecarboxamide
by
Taylor Semeniuk and Jean-Denys Hamel
Molbank 2025, 2025(4), M2078; https://doi.org/10.3390/M2078 - 21 Oct 2025
Abstract
►▼
Show Figures
Herein, the synthesis and crystallization of the unreported compound N-(2-fluoro-2-propen-1-yl)-5-(trifluoromethyl)-2-pyridinecarboxamide is achieved via amide coupling with a (2-fluoroallyl)ammonium salt. The structural properties are analyzed via single-crystal X-ray crystallography. Hydrogen bonding interactions between the amide groups and pyridine nitrogen atoms create a unique
[...] Read more.
Herein, the synthesis and crystallization of the unreported compound N-(2-fluoro-2-propen-1-yl)-5-(trifluoromethyl)-2-pyridinecarboxamide is achieved via amide coupling with a (2-fluoroallyl)ammonium salt. The structural properties are analyzed via single-crystal X-ray crystallography. Hydrogen bonding interactions between the amide groups and pyridine nitrogen atoms create a unique linear array of molecules in the crystal packing diagram. Furthermore, to validate the crystallographic data, the structural features of the compound are evaluated and compared to values reported in the literature.
Full article

Figure 1
Open AccessShort Note
2-Benzyl-6-carboxy-5,6,7,8-tetrahydroimidazo[1,2-a]pyrimidin-1-ium 2,2,2-trifluoroacetate
by
Francesco Bavo, Christos Avgerinos, Elena Martino and Bente Frølund
Molbank 2025, 2025(4), M2077; https://doi.org/10.3390/M2077 - 20 Oct 2025
Abstract
Cyclic guanidines are valuable scaffolds for the design of compounds acting on GABAergic neurotransmission, owing to their ability to mimic the amino functionality of GABA as bioisosteres. With the aim to obtain a more potent and selective betaine/GABA transporter (BGT1) inhibitor, a basic
[...] Read more.
Cyclic guanidines are valuable scaffolds for the design of compounds acting on GABAergic neurotransmission, owing to their ability to mimic the amino functionality of GABA as bioisosteres. With the aim to obtain a more potent and selective betaine/GABA transporter (BGT1) inhibitor, a basic hydrolysis of ethyl (E)-2-(acetylimino)-1-(3-phenylprop-2-yn-1-yl)hexahydropyrimidine-5-carboxylate was attempted. However, we isolated a byproduct, which was identified as the trifluoroacetate salt of 2-benzyl-5,6,7,8-tetrahydroimidazo[1,2-a]pyrimidine-6-carboxylic acid. The structure was confirmed by NMR spectroscopy and LC-MS. Herein we report the preparation, characterization, and spectral data of this fused heterocyclic compound.
Full article
(This article belongs to the Collection Molecules from Side Reactions)
►▼
Show Figures

Figure 1
Open AccessShort Note
3-((Benzyloxy)carbonyl)bicyclo[1.1.1]pentane-1-carboxylic Acid
by
Dennis D. Toporkov, Stacie K. Nelson, Jean-Denys Hamel and René T. Boeré
Molbank 2025, 2025(4), M2075; https://doi.org/10.3390/M2075 - 16 Oct 2025
Abstract
The compound 3-((benzyloxy)carbonyl)bicyclo[1.1.1]pentane-1-carboxylic acid was successfully synthesized. High-quality crystals were obtained, and its X-ray structure was solved and refined by Hirshfeld atom refinement using custom aspherical scattering factors with the Olex2/NoSphereA2 package. Hydrogen bonding interactions lead to head-to-head carboxylic acid dimer formation. A
[...] Read more.
The compound 3-((benzyloxy)carbonyl)bicyclo[1.1.1]pentane-1-carboxylic acid was successfully synthesized. High-quality crystals were obtained, and its X-ray structure was solved and refined by Hirshfeld atom refinement using custom aspherical scattering factors with the Olex2/NoSphereA2 package. Hydrogen bonding interactions lead to head-to-head carboxylic acid dimer formation. A positional disorder for the bridging H-atom was detected and modeled to two parts in a 0.85:0.15 ratio. Detailed comparison with a neutron diffraction study of benzoic acid at the same temperature (100 K) demonstrates that the E–H-bond distances in the title compound are in excellent agreement (differing less than 1%) and the displacement ellipsoids volumes to the model are also in excellent agreement to the neutron diffraction structure. Moreover, both the variation in refined disorder occupancy and differences in C=O and C–O lengths of the disordered carboxylic acids in the two structures track well with their dimer O···O separations. This is longer by 0.023 Å in the structure of the title compound than in that of benzoic acid. A database search was conducted and used for comparison of the title compound to other high-quality structures of bicyclo[1.1.1]pentane-containing species.
Full article
(This article belongs to the Section Structure Determination)
►▼
Show Figures

Figure 1
Open AccessShort Note
1,1-Bis(4-ethylphenyl)-propan-1,2-diol
by
Ichika Hayashida, Malokhat Uktamova, Sarvinoz Tirkasheva and Kohei Torikai
Molbank 2025, 2025(4), M2076; https://doi.org/10.3390/M2076 - 16 Oct 2025
Abstract
Diols represent a structurally diverse class of compounds with considerable biological and functional significance. Herein, we describe the synthesis of 1,1-bis(4-ethylphenyl)propan-1,2-diol (BEPP) via a Grignard reaction. The structure of BEPP was unambiguously elucidated by 1H and 13C nuclear magnetic resonance (NMR),
[...] Read more.
Diols represent a structurally diverse class of compounds with considerable biological and functional significance. Herein, we describe the synthesis of 1,1-bis(4-ethylphenyl)propan-1,2-diol (BEPP) via a Grignard reaction. The structure of BEPP was unambiguously elucidated by 1H and 13C nuclear magnetic resonance (NMR), heteronuclear multiple-bond correlation (HMBC), high-resolution mass spectrometry (HRMS), and infrared (IR) spectroscopy.
Full article
(This article belongs to the Section Organic Synthesis and Biosynthesis)
►▼
Show Figures

Graphical abstract
attachment
Supplementary material:
Supplementary File 1 (ZIP, 6983 KB)
Supplementary File 2 (MOL, 4 KB)
Supplementary File 3 (INCHI, 1 KB)
Supplementary File 4 (MOL, 4 KB)
Supplementary File 5 (MOL, 4 KB)
Supplementary File 6 (INCHI, 1 KB)
Supplementary File 7 (MOL, 4 KB)
Supplementary File 8 (MOL, 4 KB)
Supplementary File 9 (INCHI, 1 KB)
Supplementary File 10 (MOL, 4 KB)
Supplementary File 11 (MOL, 4 KB)
Supplementary File 12 (INCHI, 1 KB)
Supplementary File 13 (MOL, 4 KB)
Supplementary File 14 (MOL, 4 KB)
Supplementary File 15 (INCHI, 1 KB)
Supplementary File 16 (MOL, 4 KB)
Supplementary File 1 (ZIP, 6983 KB)
Supplementary File 2 (MOL, 4 KB)
Supplementary File 3 (INCHI, 1 KB)
Supplementary File 4 (MOL, 4 KB)
Supplementary File 5 (MOL, 4 KB)
Supplementary File 6 (INCHI, 1 KB)
Supplementary File 7 (MOL, 4 KB)
Supplementary File 8 (MOL, 4 KB)
Supplementary File 9 (INCHI, 1 KB)
Supplementary File 10 (MOL, 4 KB)
Supplementary File 11 (MOL, 4 KB)
Supplementary File 12 (INCHI, 1 KB)
Supplementary File 13 (MOL, 4 KB)
Supplementary File 14 (MOL, 4 KB)
Supplementary File 15 (INCHI, 1 KB)
Supplementary File 16 (MOL, 4 KB)
Open AccessCommunication
Efficient Synthesis of Unsymmetrical 7,7′-Biindolizines
by
Roxana Ciorteanu, Andreea Danila, Catalina Ionica Ciobanu, Ioana Radu, Ionel I. Mangalagiu and Ramona Danac
Molbank 2025, 2025(4), M2074; https://doi.org/10.3390/M2074 - 15 Oct 2025
Abstract
►▼
Show Figures
Six new unsymmetrical 7,7′-biindolizines were synthesized through an efficient metal-free [2+2+1] cycloaddition of ethyl 3-benzoyl-7-(pyridin-4-yl)indolizine-1-carboxylate with two equivalents of dimethyl acetylenedicarboxylate in methanol. The transformation involves one C≡C triple bond cleavage and provides access to previously unexplored unsymmetrical functionalized 7,7′-biindolizines.
Full article
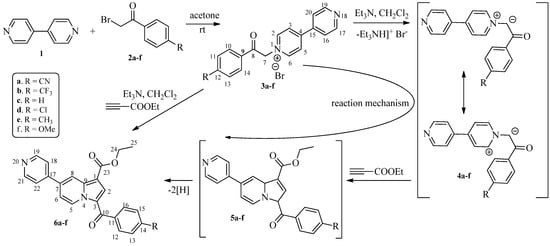
Scheme 1
Open AccessShort Note
(5R*,6R*) 11-Benzoyl-4,10-dimethyl-2,8-diphenyl-2,3,8,9-tetraazadispiro [4.0.46.15]undeca-3,9-diene-1,7-dione
by
Michail N. Elinson, Varvara M. Kalashnikova, Yuliya E. Ryzhkova and Oleg A. Rakitin
Molbank 2025, 2025(4), M2073; https://doi.org/10.3390/M2073 - 15 Oct 2025
Abstract
Cyclopropanes are important in drug discovery because their unique structure, including inherent three-dimensionality, can enhance a drug’s properties, such as metabolic stability, target binding, and membrane permeability. In this communication, (5R*,6R*) 11-benzoyl-4,10-dimethyl-2,8-diphenyl-2,3,8,9-tetraazadispiro[4.0.46.15]undeca-3,9-diene-1,7-dione was prepared via
[...] Read more.
Cyclopropanes are important in drug discovery because their unique structure, including inherent three-dimensionality, can enhance a drug’s properties, such as metabolic stability, target binding, and membrane permeability. In this communication, (5R*,6R*) 11-benzoyl-4,10-dimethyl-2,8-diphenyl-2,3,8,9-tetraazadispiro[4.0.46.15]undeca-3,9-diene-1,7-dione was prepared via a stereoselective one-pot reaction of phenylglyoxal hydrate with two equivalents of 5-methyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one in EtOH in the presence of sodium acetate and N-bromosuccinimide. The structure of the newly synthesized compound was established by 1H and 13C NMR, IR spectroscopy, high-resolution mass spectrometry, and elemental analysis.
Full article
(This article belongs to the Section Organic Synthesis and Biosynthesis)
►▼
Show Figures

Graphical abstract
attachment
Supplementary material:
Supplementary File 1 (ZIP, 3081 KB)
Supplementary File 2 (MOL, 4 KB)
Supplementary File 3 (INCHI, 1 KB)
Supplementary File 4 (MOL, 3 KB)
Supplementary File 5 (MOL, 4 KB)
Supplementary File 6 (INCHI, 1 KB)
Supplementary File 7 (MOL, 4 KB)
Supplementary File 8 (MOL, 4 KB)
Supplementary File 9 (INCHI, 1 KB)
Supplementary File 10 (MOL, 4 KB)
Supplementary File 1 (ZIP, 3081 KB)
Supplementary File 2 (MOL, 4 KB)
Supplementary File 3 (INCHI, 1 KB)
Supplementary File 4 (MOL, 3 KB)
Supplementary File 5 (MOL, 4 KB)
Supplementary File 6 (INCHI, 1 KB)
Supplementary File 7 (MOL, 4 KB)
Supplementary File 8 (MOL, 4 KB)
Supplementary File 9 (INCHI, 1 KB)
Supplementary File 10 (MOL, 4 KB)
Open AccessCommunication
Development of New Amide Derivatives of Betulinic Acid: Synthetic Approaches and Structural Characterization
by
Qinwei Xu, Yuhan Xie, Jin Qi, Zimo Ren, Carmine Coluccini and Paolo Coghi
Molbank 2025, 2025(4), M2072; https://doi.org/10.3390/M2072 - 13 Oct 2025
Abstract
In this study, we report the synthesis of three new derivatives of betulinic acid, a pentacyclic triterpenoid known for its antitumor activity. These derivatives were synthesized via amide bond formation at the C-28 position using 3-[(Ethylimino)methylidene]amino-N,N-dimethylpropan-1-amine (EDC)/Hydroxybenzotriazole (HOBt) activation
[...] Read more.
In this study, we report the synthesis of three new derivatives of betulinic acid, a pentacyclic triterpenoid known for its antitumor activity. These derivatives were synthesized via amide bond formation at the C-28 position using 3-[(Ethylimino)methylidene]amino-N,N-dimethylpropan-1-amine (EDC)/Hydroxybenzotriazole (HOBt) activation and various amines as nucleophiles. The synthesized compounds were characterized by nuclear magnetic resonance (NMR) techniques, including proton (1H), carbon-13 (13C), COSY, HSQC, and DEPT, as well as ultraviolet–visible (UV-VIS) spectroscopy, Fourier-transform infrared (IR) and elemental analysis. This work highlights the potential of semi-synthetic modification of betulinic acid to enhance anticancer properties while addressing challenges in solubility and bioavailability. Further structural optimization and formulation studies are warranted to improve drug-like properties and therapeutic applicability.
Full article
(This article belongs to the Section Organic Synthesis and Biosynthesis)
►▼
Show Figures

Figure 1
Open AccessShort Note
2,2,3,3-Tetrafluoropropyl 4-azido-2,3,5,6-Tetrafluorobenzoate
by
Sofia S. Kascheeva, Anastasiya V. Lastovka, Andrey S. Vinogradov, Tatyana V. Mezhenkova and Dmitriy A. Parkhomenko
Molbank 2025, 2025(4), M2070; https://doi.org/10.3390/M2070 - 10 Oct 2025
Abstract
Organic azides are traditionally used in organic synthesis to obtain a wide variety of chemical compounds. This prompted us to report the synthesis of a new polyfluorinated aryl azide, 2,2,3,3-tetrafluoropropyl 4-azido-2,3,5,6-tetrafluorobenzoate, which was obtained in two stages starting from pentafluorobenzoic acid.
Full article
(This article belongs to the Section Organic Synthesis and Biosynthesis)
►▼
Show Figures
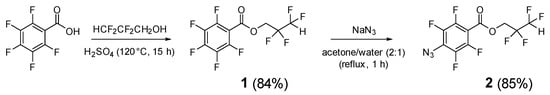
Scheme 1
Open AccessCommunication
Crystal Structure of 3-(Anthracen-2′-yl)-ortho-carborane
by
Kyrill Yu. Suponitsky, Akim V. Shmal’ko, Sergey A. Anufriev and Igor B. Sivaev
Molbank 2025, 2025(4), M2071; https://doi.org/10.3390/M2071 - 10 Oct 2025
Abstract
Crystal molecular structure of 3-(anthracen-2′-yl)-ortho-carborane was determined by single crystal X-ray diffraction study at 100 K. The asymmetric cell unit contains two enantiomeric pairs of molecules, in one of which the intramolecular dihydrogen bond CH...HB is formed with the participation of
[...] Read more.
Crystal molecular structure of 3-(anthracen-2′-yl)-ortho-carborane was determined by single crystal X-ray diffraction study at 100 K. The asymmetric cell unit contains two enantiomeric pairs of molecules, in one of which the intramolecular dihydrogen bond CH...HB is formed with the participation of the C(1)H hydrogen of the anthracene substituent, and in the other with the participation of the C(3)H hydrogen. In all molecules, the polycyclic aromatic and carborane fragments are rotated relative to each other in such a way that the C-C bond of the ortho-carborane cage is approximately parallel to the plane of the aromatic substituent. According to quantum chemical calculations, the minimum energy corresponds to the formation of an intramolecular dihydrogen bond C(1)H...HB(4/7), whereas the C(3)H...HB(4/7) bond is formed rather as a result of intermolecular interactions in the crystal lattice.
Full article
(This article belongs to the Section Structure Determination)
►▼
Show Figures

Figure 1
Open AccessCommunication
Gas Phase Fragmentation of N,N-Ditosyl-2-aminodiphenylamine to Phenazine
by
M. John Plater and William T. A. Harrison
Molbank 2025, 2025(4), M2069; https://doi.org/10.3390/M2069 - 6 Oct 2025
Abstract
N,N-Ditosyl-2-aminodiphenylamine was prepared by the tosylation of 2-aminodiphenylamine with tosylchloride in dichloromethane. Unwanted isomers owing to the tosylation of the diarylamine were not formed. This compound was fully characterized by IR, UV/Vis, NMR, m/z, and mp, including an X-Ray
[...] Read more.
N,N-Ditosyl-2-aminodiphenylamine was prepared by the tosylation of 2-aminodiphenylamine with tosylchloride in dichloromethane. Unwanted isomers owing to the tosylation of the diarylamine were not formed. This compound was fully characterized by IR, UV/Vis, NMR, m/z, and mp, including an X-Ray single crystal structure determination. It was fragmented in an Atmospheric Solids Analysis Probe (ASAP) mass spectrometer showing a series of fragments down to phenazine.
Full article
(This article belongs to the Section Organic Synthesis and Biosynthesis)
►▼
Show Figures

Figure 1
attachment
Supplementary material:
Supplementary File 1 (ZIP, 2382 KB)
Supplementary File 2 (MOL, 2 KB)
Supplementary File 3 (INCHI, 688 B)
Supplementary File 4 (MOL, 2 KB)
Supplementary File 5 (MOL, 2 KB)
Supplementary File 6 (INCHI, 718 B)
Supplementary File 7 (MOL, 2 KB)
Supplementary File 8 (MOL, 2 KB)
Supplementary File 9 (INCHI, 625 B)
Supplementary File 10 (MOL, 2 KB)
Supplementary File 1 (ZIP, 2382 KB)
Supplementary File 2 (MOL, 2 KB)
Supplementary File 3 (INCHI, 688 B)
Supplementary File 4 (MOL, 2 KB)
Supplementary File 5 (MOL, 2 KB)
Supplementary File 6 (INCHI, 718 B)
Supplementary File 7 (MOL, 2 KB)
Supplementary File 8 (MOL, 2 KB)
Supplementary File 9 (INCHI, 625 B)
Supplementary File 10 (MOL, 2 KB)
Open AccessCommunication
Synthesis and Characterization of Novel Pyridinium Salts of (E)-2-(Pyridin-4-ylmethylene)hydrazine-1-carboximidamide
by
Fatemeh Ataie Alani, Fatemeh Ahmadian, Alireza Houshdar Tehrani and Salimeh Amidi
Molbank 2025, 2025(4), M2068; https://doi.org/10.3390/M2068 - 1 Oct 2025
Abstract
We report the synthesis and characterization of the novel pyridinium salts from (E)-2-(pyridin-4-ylmethylene)hydrazine-1-carboximidamide. The pyridinium salts were obtained via the reaction of guanylhydrazone derived from pyridine-4-carbaldehyde with phenacyl bromides. Structural characterization was carried out using IR, 1H, and 13C
[...] Read more.
We report the synthesis and characterization of the novel pyridinium salts from (E)-2-(pyridin-4-ylmethylene)hydrazine-1-carboximidamide. The pyridinium salts were obtained via the reaction of guanylhydrazone derived from pyridine-4-carbaldehyde with phenacyl bromides. Structural characterization was carried out using IR, 1H, and 13C NMR spectroscopy and mass spectrometry.
Full article
(This article belongs to the Section Organic Synthesis and Biosynthesis)
►▼
Show Figures

Scheme 1
Open AccessShort Note
3-(4-Hydroxy-2,3,5-trimethylphenyl)-3-(4-hydroxyphenyl)isobenzofuran-1(3H)-one
by
Brian A. Chalmers, David B. Cordes, Aidan P. McKay, Iain L. J. Patterson, Russell J. Pearson, Nadiia Vladymyrova and Iain A. Smellie
Molbank 2025, 2025(4), M2067; https://doi.org/10.3390/M2067 - 1 Oct 2025
Abstract
3-(4-hydroxy-2,3,5-trimethylphenyl)-3-(4-hydroxyphenyl)isobenzofuran-1(3H)-one is a derivative of the well-known acid/base indicator, phenolphthalein. We report the synthesis and the molecular structure of the title compound as determined by single-crystal X-ray diffraction. 1H and 13C NMR spectroscopy, IR spectroscopy, and mass spectrometry data have been provided.
Full article
(This article belongs to the Section Structure Determination)
►▼
Show Figures

Figure 1
Open AccessShort Note
Dichloro[2,5-bis(diisopropylphosphorimidoyl-κN-(4,6-dimethylpyrimidine-κN))pyrrole-κN]yttrium(III)·toluene
by
Emily L. Trew, David Szucs and Paul G. Hayes
Molbank 2025, 2025(4), M2066; https://doi.org/10.3390/M2066 - 30 Sep 2025
Abstract
►▼
Show Figures
The compound dichloro[bis(diisopropylphosphorimidoyl-κN-(4,6-dimethylpyrimidine-κN))pyrrole-κN]yttrium(III) was synthesized from one equivalent of NaL [L = 2,5-[iPr2P=N(PymMe)]2NH(C4H2); PymMe = 4,6-dimethylpyrimidine] and YCl3(THF)3.5 and crystallized from
[...] Read more.
The compound dichloro[bis(diisopropylphosphorimidoyl-κN-(4,6-dimethylpyrimidine-κN))pyrrole-κN]yttrium(III) was synthesized from one equivalent of NaL [L = 2,5-[iPr2P=N(PymMe)]2NH(C4H2); PymMe = 4,6-dimethylpyrimidine] and YCl3(THF)3.5 and crystallized from toluene. X-ray quality crystals of LYCl2 were obtained with one toluene solvent molecule in the asymmetric unit. The geometry, bond lengths and angles were analyzed and found to contain similar parameters to comparable structures in the literature, and the product was further characterized by NMR spectroscopy. To the best of our knowledge, this is the first reported seven-coordinate Y(III) complex supported by a pentadentate ligand wherein all five donor atoms are nitrogen.
Full article

Figure 1
Open AccessShort Note
(±)-2-(4-Isobutylphenyl)-N-(naphthalen-1-yl)propanamide
by
Diyana Dimitrova, Iliyan Ivanov, Stanimir Manolov and Dimitar Bojilov
Molbank 2025, 2025(4), M2065; https://doi.org/10.3390/M2065 - 30 Sep 2025
Abstract
We describe the synthesis of (±)-2-(4-isobutylphenyl)-N-(naphthalen-1-yl)propanamide, followed by comprehensive structural characterization. The compound was analyzed through melting point determination, 1H and 13C NMR spectroscopy, infrared spectroscopy, and mass spectrometry. The concordant results from these techniques provide clear evidence for
[...] Read more.
We describe the synthesis of (±)-2-(4-isobutylphenyl)-N-(naphthalen-1-yl)propanamide, followed by comprehensive structural characterization. The compound was analyzed through melting point determination, 1H and 13C NMR spectroscopy, infrared spectroscopy, and mass spectrometry. The concordant results from these techniques provide clear evidence for the successful preparation and structural confirmation of the target molecule.
Full article
(This article belongs to the Section Structure Determination)
►▼
Show Figures

Figure 1
attachment
Supplementary material:
Supplementary File 1 (ZIP, 1335 KB)
Supplementary File 2 (MOL, 2 KB)
Supplementary File 3 (INCHI, 545 B)
Supplementary File 4 (MOL, 2 KB)
Supplementary File 5 (MOL, 2 KB)
Supplementary File 6 (INCHI, 624 B)
Supplementary File 7 (MOL, 1 KB)
Supplementary File 8 (MOL, 1 KB)
Supplementary File 9 (INCHI, 414 B)
Supplementary File 10 (MOL, 1 KB)
Supplementary File 11 (CIF, 188 KB)
Supplementary File 12 (CIF, 324 KB)
Supplementary File 13 (CIF, 260 KB)
Supplementary File 1 (ZIP, 1335 KB)
Supplementary File 2 (MOL, 2 KB)
Supplementary File 3 (INCHI, 545 B)
Supplementary File 4 (MOL, 2 KB)
Supplementary File 5 (MOL, 2 KB)
Supplementary File 6 (INCHI, 624 B)
Supplementary File 7 (MOL, 1 KB)
Supplementary File 8 (MOL, 1 KB)
Supplementary File 9 (INCHI, 414 B)
Supplementary File 10 (MOL, 1 KB)
Supplementary File 11 (CIF, 188 KB)
Supplementary File 12 (CIF, 324 KB)
Supplementary File 13 (CIF, 260 KB)
Open AccessCommunication
A Tandem Photocycloaddition—Ring Expansion Strategy for the Synthesis of Fused [5.3.0] Triketone
by
Xin-Yi Hsiao, Chern Chuang and Gary Jing Chuang
Molbank 2025, 2025(3), M2064; https://doi.org/10.3390/M2064 - 22 Sep 2025
Abstract
A tandem synthetic sequence involving photo-induced intramolecular [2+2] cycloaddition followed by acid-promoted ring expansion was developed to access the novel bicyclic triketone framework. The process begins with the UV (254 nm) irradiation of cyclic vinylogous ester, affording a highly strained cyclobutane-fused diketone in
[...] Read more.
A tandem synthetic sequence involving photo-induced intramolecular [2+2] cycloaddition followed by acid-promoted ring expansion was developed to access the novel bicyclic triketone framework. The process begins with the UV (254 nm) irradiation of cyclic vinylogous ester, affording a highly strained cyclobutane-fused diketone in an 86% yield. This unique intermediate feature is of a fused four- and six-membered ring system with spatially compressed carbonyl groups. Upon acidic hydrolysis in aqueous MeCN, the strained system undergoes retro-aldol ring expansion, delivering [5.3.0] bicyclic triketones bearing a seven- and five-membered fused ring with three strategically oriented carbonyl units in a 75% yield. Structural elucidation was performed using NMR spectroscopy, UV-Vis, HRMS, and single-crystal X-ray crystallography. The method highlights a concise route for constructing a fused bicyclic triketone of relevance to synthetic and medicinal chemistry.
Full article
(This article belongs to the Section Organic Synthesis and Biosynthesis)
►▼
Show Figures

Figure 1
Open AccessShort Note
1,1,1,3,3,3-Hexafluoropropan-2-yl 2,3,5,6-tetrafluoro-4-((1,1,1,3,3,3-hexafluoropropan-2-yl)oxy)benzoate
by
Sofia S. Kascheeva, Anastasiya V. Lastovka, Andrey S. Vinogradov and Dmitriy A. Parkhomenko
Molbank 2025, 2025(3), M2063; https://doi.org/10.3390/M2063 - 18 Sep 2025
Abstract
Acyl chloride alcoholysis is a fundamental and typically high-yielding method for ester synthesis. However, competitive side reactions can occur when the acyl chloride possesses multiple electrophilic sites and the alcohol is a strong nucleophile. We report an example of this phenomenon: the reaction
[...] Read more.
Acyl chloride alcoholysis is a fundamental and typically high-yielding method for ester synthesis. However, competitive side reactions can occur when the acyl chloride possesses multiple electrophilic sites and the alcohol is a strong nucleophile. We report an example of this phenomenon: the reaction of pentafluorobenzoyl chloride with 1,1,1,3,3,3-hexafluoropropan-2-ol yields not only the expected ester but also a significant quantity of the 1,1,1,3,3,3-hexafluoropropan-2-yl 2,3,5,6-tetrafluoro-4-((1,1,1,3,3,3-hexafluoropropan-2-yl)oxy)benzoate. The formation of the latter results from an effective nucleophilic aromatic substitution (SNAr) at the para-fluorine position of the pentafluorophenyl ring by the hexafluoroisopropoxide anion.
Full article
(This article belongs to the Collection Molecules from Side Reactions)
►▼
Show Figures

Scheme 1
attachment
Supplementary material:
Supplementary File 1 (ZIP, 586 KB)
Supplementary File 2 (MOL, 3 KB)
Supplementary File 4 (MOL, 3 KB)
Supplementary File 1 (ZIP, 586 KB)
Supplementary File 2 (MOL, 3 KB)
Supplementary File 4 (MOL, 3 KB)
Open AccessShort Note
1-((Dimethyl(3-((2,2,3,3,4,4,5,5,6,6,7,7,8,8,8-pentadecafluorooctyl)oxy)propyl)silyl)oxy)-3,5,7,9,11,13,15-heptakis((dimethylsilyl)oxy)-octasilsesquioxane
by
Analise C. H. Migliaccio, Andrea R. Kelley and Scott T. Iacono
Molbank 2025, 2025(3), M2062; https://doi.org/10.3390/M2062 - 17 Sep 2025
Abstract
The title compound was synthesized using Pt-catalyzed hydrosilylation of octasilane POSS and allyl 1H,1H-perfluorooctyl ether. The purity and structure were determined by NMR (1H, 13C, 19F, 29Si), and MALDI TOF-MS.
Full article
(This article belongs to the Section Organic Synthesis and Biosynthesis)
►▼
Show Figures

Scheme 1
Open AccessShort Note
(R)-4-Acetyl-10-(2-chloro-1,3-thiazol-4-yl)-5,11,13-trihydroxy-2,12-dimethyl-8-oxatricyclo[7.4.0.02,7]trideca-1(13),4,6,9,11-pentaen-3-one
by
Aleksandr S. Filimonov, Olga A. Luzina and Nariman F. Salakhutdinov
Molbank 2025, 2025(3), M2061; https://doi.org/10.3390/M2061 - 16 Sep 2025
Abstract
►▼
Show Figures
A novel usnic acid derivative with 2-chlorothiazole substituent was obtained by a three-step synthesis from usnic acid. The structure of the product was proved by a set of physical methods, including 1H, 13C, HRMS, HSQC, HMBC and IR spectroscopy.
Full article

Figure 1
Highly Accessed Articles
Latest Books
E-Mail Alert
News
Topics
Topic in
Catalysts, Chemistry, Molbank, Molecules, Sustainable Chemistry
Towards the Sustainable Synthesis of Biologically Active Molecules in Green Solvents
Topic Editors: Antonio Salomone, Serena PerroneDeadline: 31 December 2025

Conferences
Special Issues
Special Issue in
Molbank
Carbonylation Chemistry in the Synthesis of High Value Added Compounds
Guest Editors: Bartolo Gabriele, Raffaella MancusoDeadline: 20 April 2026
Topical Collections
Topical Collection in
Molbank
Molecules from Catalytic Processes
Collection Editor: Nicola Della Ca’