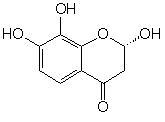
(S*)-2,7,8-Trihydroxychroman-4-one
Abstract
:1. Introduction
2. Results and Discussion
3. Experimental
3.1. General
3.2. Plant Material
3.3. Extraction and Isolation
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgments
References
- Kuo, Y.H.; Shue, M.J. New esters, 2-(4-hydroxy-3-methoxyphenyl)ethyl hexa- and octacosanoates from the leaves of Cinnamomum reticulatum Hay. J. Chin. Chem. Soc. 1991, 38, 65–69. [Google Scholar] [CrossRef]
- Lin, R.J.; Cheng, M.J.; Huang, J.C.; Lo, W.L.; Yeh, Y.T.; Yen, C.M.; Lu, C.M.; Chen, C.Y. Cytotoxic compounds from the stems of Cinnamomum tenuifolium. J. Nat. Prod. 2009, 72. in press. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Chen, C.H.; Lo, Y.C.; Wu, B.N.; Wang, H.M.; Lo, W.L.; Yen, C.M.; Lin, R.J. Anticancer activity of Isoobtusilactone A from Cinnamomum kotoense: Involvement of apoptosis, cell-cycle dysregulation, mitochondria regulation, and reactive oxygen species. J. Nat. Prod. 2008, 71, 933–940. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Hsu, Y.L.; Tsai, Y.C.; Kuo, P.L. Kotomolide A arrests cell cycle progression and induces apoptosis through the induction of ATM/p53 and the initiation of mitochondrial system in human non-small cell lung cancer A549 cells. Food Chem. Toxicol. 2008, 46, 2476–2484. [Google Scholar] [CrossRef] [PubMed]
- Kuo, P.L.; Chen, C.Y.; Tzeng, T.F.; Lin, C.C.; Hsu, Y.L. Involvement of reactive oxygen species/c-Jun NH2-terminal kinase pathway in kotomolide A induces apoptosis in human breast cancer cells. Toxicol. Appl. Pharmacol. 2008, 229, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Kuo, S.Y.; Hsieh, T.J.; Wang, Y.D.; Lo, W.L.; Hsui, Y.R.; Chen, C.Y. Cytotoxic constituents from the leaves of Cinnamomum subavenium. Chem. Pharm. Bull. 2008, 56, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.Z.; Cheng, J.T.; Yiin, S.J.; Chen, C.Y.; Chen, C.H.; Chen, C.H. Isoobtusilactone A induces both caspase–dependent and –independent apoptosis in Hep G2 cells. Food Chem. Toxicol. 2008, 46, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.J.; Lo, W.L.; Wang, Y.D.; Chen, C.Y. A novel cytotoxic monoterpenoid from the leaves of Cinnamomum subavenium. Nat. Prod. Res. 2008, 22, 1055–1059. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Chen, C.H.; Wong, C.H.; Liu, Y.W.; Lin, Y.S.; Wang, Y.D.; Hsui, Y.R. Cytotoxic constituents of the stems of Cinnamomum subavenium. J. Nat. Prod. 2007, 70, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Hsu, Y.L.; Chen, Y.Y.; Hung, J.Y.; Huang, M.S.; Kuo, P.L. Isokotomolide A, a new butanolide extracted from the leaves of Cinnamomum kotoense, arrests cell cycle progression and induces apoptosis through the induction of p53/p21 and the initiation of mitochondrial system in human non-small cell lung cancer A549 cells. Eur. J. Pharmacol. 2007, 574, 94–102. [Google Scholar] [PubMed]
- Chen, C.Y.; Liu, T.Z.; Chen, C.H.; Wu, C.C.; Cheng, J.T.; Yiin, S.J.; Shih, M.K.; Wu, M.J.; Chern, C.L. Isoobtusilactone A-induced apoptosis in human hepatoma Hep G2 cells is mediated via increased NADPH oxidase-derived reactive oxygen species (ROS) production and the mitochondria-associated apoptotic mechanisms. Food Chem. Toxicol. 2007, 45, 1268–1276. [Google Scholar] [CrossRef] [PubMed]
- Kuo, P.L.; Chen, C.Y.; Hsu, Y.L. Isoobtusilactone A induced cell cycle arrest and apoptosis through reactive oxygen species/apoptosis signal-regulating kinase 1 signaling pathway in human breast cancer cells. Cancer Res. 2007, 67, 7406–7420. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Lo, W.L.; Liu, Y.C.; Chen, C.Y. Chemical and cytotoxic constituents from the leaves of Cinnamomum kotoense. J. Nat. Prod. 2006, 69, 927–933. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y. Butanolides from the stem of Cinnamomum kotoense. Nat. Prod. Commun. 2006, 1, 453–455. [Google Scholar]
- Hsieh, T.J.; Chen, C.H.; Lo, W.L.; Chen, C.Y. Lignans from the stem of Cinnamomum camphora. Nat. Prod. Commun. 2006, 1, 21–25. [Google Scholar]
- Hsieh, T.J.; Su, C.C.; Chen, C.Y.; Liou, C.H.; Lu, L.H. Using experimental studies and theoretical calculations to analyze the molecular mechanism of coumarin, p-hydroxybenzoic acid, and cinnamic acid. J. Mol. Struct. 2005, 741, 21–25. [Google Scholar] [CrossRef]
- Chen, C.Y.; Hsieh, S.L.; Hsieh, M.M.; Hsieh, S.F.; Hsieh, T.J. Substituent chemical shift of rhamnosides from the stems of Cinnamomum osmophleum. Chin. Pharm. J. 2004, 56, 141–146. [Google Scholar]
- Ngo, L.V.; Thi, C.P. An unusual m-hydroxyacetophenone and three new chromanone derivatives from Chrysothamnus viscidiflorus. Phytochemistry 1981, 20, 485–487. [Google Scholar] [CrossRef]


© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Cheng, M.-J.; Lo, W.-L.; Yeh, H.-C.; Chen, C.-Y. (S*)-2,7,8-Trihydroxychroman-4-one. Molbank 2009, 2009, M626. https://doi.org/10.3390/M626
Cheng M-J, Lo W-L, Yeh H-C, Chen C-Y. (S*)-2,7,8-Trihydroxychroman-4-one. Molbank. 2009; 2009(4):M626. https://doi.org/10.3390/M626
Chicago/Turabian StyleCheng, Ming-Jen, Wen-Li Lo, Hung-Chun Yeh, and Chung-Yi Chen. 2009. "(S*)-2,7,8-Trihydroxychroman-4-one" Molbank 2009, no. 4: M626. https://doi.org/10.3390/M626
APA StyleCheng, M.-J., Lo, W.-L., Yeh, H.-C., & Chen, C.-Y. (2009). (S*)-2,7,8-Trihydroxychroman-4-one. Molbank, 2009(4), M626. https://doi.org/10.3390/M626