Abstract
Methyl 6-methyl-4-(4-iodophenyl)-1,2,3,4-tetrahydro-2-thioxo-5-pyrimidine-carboxylate has been synthesized via Biginelli reaction of 4-iodobenzaldehyde, methyl acetoacetate and thiourea, promoted by microwave irradiation in the presence of iodine under solvent-free conditions in high yield and good purity.
In recent years, dihydropyrimidinones (DHPMs) and their derivatives have attracted considerable attention in natural and synthetic organic chemistry because of their biological and medicinal properties [1,2]. The venerable Biginelli reaction [3,4], one-pot cyclocondensation of aldehyde, β-ketoester, and urea or thiourea, is inarguably one of the most useful multi-component reactions. Polyfunctionalized dihydropyrimidines represent a heterocyclic system of remarkable pharmacological properties such as antiviral, antitumor, antibacterial, and antiinflammatory properties.
Because of global environmental concern and social sustainable development, green synthesis has received considerable attention [5]. Molecular iodine as an inexpensive, nontoxic, readily available catalyst has been used successfully in the Biginelli reaction [6,7], affording the corresponding products in excellent yields with high selectivity [6,7,8,9]. Microwave-assisted chemistry has also attracted a considerable attention in recent years and has been applied successfully in various fields of synthetic organic chemistry [10,11,12,13], including solvent-free reactions [12,13].
As our continuous investigation on the methodology of green synthesis, we report herein the synthesis of the new DHPM compound, methyl 6-methyl-4-(4-iodophenyl)-1,2,3,4-tetrahydro-2-thioxo-5-pyrimidinecarboxylate via a Biginelli three-component cyclocondensation of 4-iodobenz-aldehyde, methyl acetoacetate and thiourea, catalyzed with iodine and promoted by microwave irradiation (MWI) under solvent-free conditions (Scheme 1). The title compound has been fully characterized by NMR (1H and 13C), IR, MS, and elemental analysis. This protocol is proven to be efficient and environmentally benign.
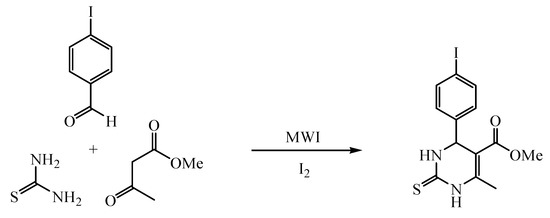
Scheme 1.
Experimental Procedure
4-Iodobenzaldehyde (2.5 mmol), methyl acetoacetate (5 mmol), thiourea (5 mmol) and iodine (0.2 mmol) were mixed thoroughly and irradiated in a microwave reactor (600 W) at 60 °C for 15 min and monitored by TLC. After completion of the reaction, the reaction mixture was poured into ethyl acetate, cooled to precipitate out, and filtered off to obtain the crude product in high yield. Recrystallization from ethanol afforded the pure title compound as yellow crystals in a yield of 87.7%, m.p. 187.9–189.2 °C.
Moreover, our investigation showed that the best results were observed when the molar ratio of aldehyde, acetoacetate and thiourea was 1:2:2. In addition, it was found that the yields were not obviously affected by different amount of iodine.
1H NMR (Bruker 300 MHz, DMSO-d6): δH 10.38 (s, 1H, N-H), 9.66 (s, 1H, N-H), 7.71 (d, J = 8.0 Hz, 2H, Ar-H), 7.01 (d, J = 8.1 Hz, 2H, Ar-H), 5.11 (d, J = 3.0 Hz, 1H, C4-H), 3.54 (s, 3H, OCH3), 2.29 (s, 3H, CH3) ppm.
13C NMR (75 MHz, DMSO-d6): δC 174.8, 165.9, 146.0, 143.5, 137.9, 129.1, 100.5, 94.3, 54.0, 51.6, 17.7 ppm.
IR (Bruker Tensor 27, KBr): νmax 3453, 2361, 1662, 1576, 1196, 1118 cm-1.
MS (API 4000, ESI): m/z (%) 389.2 (M+, base peak).
Elemental anal. (Perkin Elmer PE 2400 II HONS): calcd for C13H13IN2O2S: C 40.21, H 3.37, N 7.20; found: C 40.22, H 3.38, N 7.22.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgements
The authors are grateful to Liu Zhixian and Yu Lixin for determinations of NMR, IR and elemental analysis.
References
- Kappe, C.O. 100 Years of Biginelli dihydropyrimidine synthesis. Tetrahedron 1993, 49, 6937–6963. [Google Scholar] [CrossRef]
- Kappe, C.O. Recent advances in the Biginelli dihydropyrimidine synthesis. New tricks from an old dog. Acc. Chem. Res. 2000, 33, 879–888. [Google Scholar] [CrossRef] [PubMed]
- Biginelli, P. Aldehyde−urea derivatives of aceto-and oxaloacetic acids. Gazz. Chim. Ital. 1893, 23, 360–413. [Google Scholar]
- Sanjeev, P.; Gokavi, G.S. Heteropoly acid catalyzed synthesis of 3,4-dihydropyrimidin-2(1H)-ones. Catal. Commun. 2007, 8, 279–284. [Google Scholar]
- Walsh, P.J.; Li, H.M.; de Parrodi, C.A. A green chemistry approach to asymmetric catalysis: solvent-free and highly concentrated reactions. Chem. Rev. 2007, 107, 2503–2545. [Google Scholar] [CrossRef] [PubMed]
- Saxena, I.; Borah, D.C.; Sarma, J.C. Three component condensations catalyzed by iodine–alumina for the synthesis of substituted 3,4-dihydropyrimidin-2(1H)-ones under microwave irradiation and solvent-free condition. Tetrahedron Lett. 2005, 46, 1159–1160. [Google Scholar] [CrossRef]
- Bhosale, R.S.; Bhosale, S.V.; Bhosale, S.V.; Wang, T.; Zubaidha, P.K. An efficient, high yield protocol for the one-pot synthesis of dihydropyrimidin-2(1H)-ones catalyzed by iodine. Tetrahedron Lett. 2004, 45, 9111–9113. [Google Scholar] [CrossRef]
- Ko, S.; Sastry, M.N.V.; Lin, C.; Yao, C.-F. Molecular iodine-catalyzed one-pot synthesis of 4-substituted-1,4-dihydropyridine derivatives via Hantzsch reaction. Tetrahedron Lett. 2005, 46, 5771–5774. [Google Scholar] [CrossRef]
- Srinivas, K.V.N.S.; Das, B. Iodine-catalyzed one-pot synthesis of 3,4-dihydropyrimidin-2(1H)-ones and thiones: A simple and efficient procedure for the Biginelli reaction. Synthesis 2004, 13, 2091–2093. [Google Scholar]
- de la Hoz, A.; Diaz-Ortiz, A.; Moreno, A.; Langa, F. Cycloadditions under microwave irradiation conditions: Methods and applications. Eur. J. Org. Chem. 2000, 3659–3673. [Google Scholar] [CrossRef]
- Varma, R.S. Solvent-free synthesis of heterocyclic compounds using microwaves. J. Heterocycl. Chem. 1999, 36, 1565–1571. [Google Scholar] [CrossRef]
- Varma, R.S. Solvent-free organic syntheses using supported reagents and microwave irradiation. Green Chem. 1999, 1, 43–55. [Google Scholar] [CrossRef]
- Loupy, A.; Petit, A.; Hamelin, J.; Texier-Boullet, F.; Jacquault, P.; Mathe, D. New solvent-free organic synthesis using focused microwaves. Synthesis 1998, 1213–1234. [Google Scholar] [CrossRef]
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).