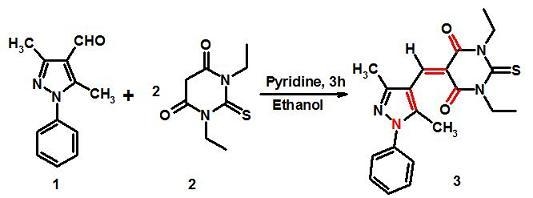
5-[(3,5-Dimethyl-1-phenyl-1H-pyrazol-4-yl)methylene]-1,3-diethyl-2-thioxodihydropyrimidine-4,6(1H,5H)-dione
Abstract
:
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgements
References and Notes
- Asiri, A.M. Synthesis and characterization of dyes exemplified by 2-arylidene-dicyanomethyleneindane. Dyes Pigm. 1999, 42, 209–213. [Google Scholar] [CrossRef]
- Jiang, L.; Chang, Q.; Ouyang, Q.; Liu, H.; Wang, Y.; Zhang, X.; Song, Y.; Li, Y. Fabrication and nonlinear optical properties of an ultrathin film with acceptor–donor periodically overlapping structure. Chem. Phys. 2006, 324, 556–562. [Google Scholar] [CrossRef]
- Wu, C.; Tretiak, S.; Chernyak, V.Y. Excited states and optical response of a donor–acceptor substituted polyene: A TD-DFT study. Chem. Phy. Lett. 2007, 433, 305–311. [Google Scholar] [CrossRef]
- Chandrassekarn, Y.; Dutta, G.K.; Kanth, R.B.; Patil, S. Tetrahydroquinoxaline based squaraines: Synthesis and photophysical properties. Dyes Pigm. 2009, 83, 162–167. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, J.; Zhang, X.; Liu, Z.; Wan, X.; Tian, J.; Wang, T.; Chen, Y. Synthesis, characterization and optical limiting property of covalently oligothiophene-functionalized graphene material. Carbon 2009, 47, 3113–3121. [Google Scholar] [CrossRef]
- Bosch, P.; Peinado, C.; Martin, V.; Catalina, F.; Corrales, T. Fluorescence monitoring of photoinitiated polymerization reactions: Synthesis, photochemical study and behaviour as fluorescent probes of new derivatives of 4′-dimethylaminostyryldiazines. J. Photochem. Photobio. A: Chem. 2006, 180, 118–129. [Google Scholar] [CrossRef]
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Asiri, A.M.; Khan, S.A. 5-[(3,5-Dimethyl-1-phenyl-1H-pyrazol-4-yl)methylene]-1,3-diethyl-2-thioxodihydropyrimidine-4,6(1H,5H)-dione. Molbank 2010, 2010, M666. https://doi.org/10.3390/M666
Asiri AM, Khan SA. 5-[(3,5-Dimethyl-1-phenyl-1H-pyrazol-4-yl)methylene]-1,3-diethyl-2-thioxodihydropyrimidine-4,6(1H,5H)-dione. Molbank. 2010; 2010(1):M666. https://doi.org/10.3390/M666
Chicago/Turabian StyleAsiri, Abdullah Mohamed, and Salman A. Khan. 2010. "5-[(3,5-Dimethyl-1-phenyl-1H-pyrazol-4-yl)methylene]-1,3-diethyl-2-thioxodihydropyrimidine-4,6(1H,5H)-dione" Molbank 2010, no. 1: M666. https://doi.org/10.3390/M666
APA StyleAsiri, A. M., & Khan, S. A. (2010). 5-[(3,5-Dimethyl-1-phenyl-1H-pyrazol-4-yl)methylene]-1,3-diethyl-2-thioxodihydropyrimidine-4,6(1H,5H)-dione. Molbank, 2010(1), M666. https://doi.org/10.3390/M666