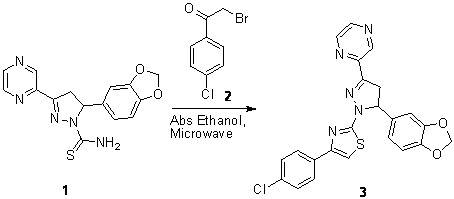
2-{5-(1,3-Benzodioxol-5-yl)-1-[4-(4-chlorophenyl)-1,3-thiazol-2-yl]-4,5-dihydro-1H-pyrazol-3-yl}pyrazine
Abstract
:Introduction

Experimental
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgements
References and Notes
- Silverman, R.B. Organic Chemistry of Drug Design and Drug Action; Academic Press: San Diego, CA, USA, 1992. [Google Scholar]
- Thompson, L.A.; Ellman, J.A. Synthesis and Applications of small molecule libraries. Chem. Rev. 1996, 96, 555–600. [Google Scholar] [CrossRef] [PubMed]
- Jungheim, L.N.; Sigmund, S.K.; Fisher, J.W. Bicyclic pyrazolidinones, a new class of antibacterial agent based on the β-lactam model. Tetrahedron Lett. 1987, 28, 285–288. [Google Scholar] [CrossRef]
- Jungheim, L.N.; Sigmund, S.K.; Jones, N.D.; Swartzendruber, J.K. Bicyclic pyrazolidinones, steric electronic effects on antibacterial activity. Tetrahedron Lett. 1987, 28, 289–292. [Google Scholar] [CrossRef]
- Boyd, D.B.; Morin, R.B.; Gorman, M. Theoretical and Physicochemical studies on β-Lactam Antibiotics in β-Lactam Antibiotics, Chemistry and Biology; Academic press: New York, NY, USA, 1982; Volume 1, pp. 437–545. [Google Scholar]
- Jungheim, L.N.; Holmes, R.E.; Ott, J.L.; Ternansky, R.J.; Draheim, S.E.; Neel, D.A.; Stepherd, T.A.; Sigmund, S.K. Abstracts of 26th Interscience Conference on Antimicrobial Agents and Chemotherapy, New Orleans, LA, USA, 28 September–1 October 1988. Paper 601.
- Jungheim, L.N.; Holmes, R.E.; Ternansky, R.J.; Stepherd, T.A.; Neel, D.A.; Draheim, S.E.; Pike, A.J.; Wu, C.Y.E. Abstracts of 28th Interscience Conference on Antimicrobial Agents and Chemotherapy, Los Angels, CA, USA, 23–26 October 1988. paper 240.
- Ternansky, R.J.; Draheim, S.E. [3.3.0] Pyrazolidinones: An efficient synthesis of a new class of synthetic antibacterial agents. Tetrahedron Lett. 1990, 31, 2805–2808. [Google Scholar] [CrossRef]
- Sangwan, N.K.; Dhindsa, K.S.; Malik, O.P.; Malik, M.S. Chim. Acta Turc. 1983, 11, 65–72.
- Safak, C.; Tayhan, A.; Sarac, S. Synthesis of some 1-Acetyl-3,5-diaryl-2-pyrazoline derivatives and their antimicrobial activities. J. Indian Chem. Soc. 1990, 67, 571–574. [Google Scholar] [CrossRef]
- Nauduri, D.; Reddy, G.B. Antibacterials and Antimycotics: Part 1: Synthesis and Activity of 2-Pyrazoline Derivatives. Chem. Pharm. Bull. (Tokyo) 1998, 46, 1254–1260. [Google Scholar] [CrossRef] [PubMed]
- Grant, N.; Mishriky, N.; Asaad, F.M.; Fawzy, N.G. Pyridines and pyrazolines from salicylic acid derivatives with propenone residue and their antimicrobial properties. Pharmazie 1998, 53, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Turan-Zitouni, G.; Özdemir, A.; Güven, K. Synthesis of some 1-[(N,N-disubstituted thiocarbamoylthio)acetyl]-3-(2-thienyl)-5-aryl-2-pyrazoline derivatives and investigation of their antibacterial antifungal activities. Arch. Pharm. Pharm. Med. Chem. 2005, 338, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Turan-Zitouni, G.; Özdemir, A.; Kaplancıkli, Z.A.; Chevallet, P.; Tunali, Y. Synthesis and antimicrobial activities of some 1-[ (N,N-disubstituted thiocarbamoylthio)acetyl]-3,5,diaryl-2- pyrazolines. Phosphorus Sulfur Silicon Relat. Elem. 2005, 180, 2717–2724. [Google Scholar] [CrossRef]
- Nasar, M.N.A.; Said, S.A. Novel 3,3a,4,5,6,7- Hexahydroindazole and arylthiazolyl pyrazoline derivatives of anti- inflammatory agents. Arch. Pharm. Pharm. Med. Chem. 2003, 336, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Turan-Zitouni, G.; Chevallet, P.; Kiliç, F.S.; Erol, K. Synthesis of some thiazolyl- pyrazoline derivatives and preliminary investigation of their hypotensive activity. Eur. J. Med. Chem. 2000, 35, 635–641. [Google Scholar] [CrossRef]
- Gupta, R.R.; Kumar, M.; Gupta, V. Heterocyclic Chemistry Five- membered Heterocycles; Springer- Verlag: Berlin, Heidelberg, New York, 1999; Volume 2, p. 416. [Google Scholar]
- Onoe, H.; Takahashi, Jpn. Kokai. Tokyo Koho JP 03 87,841. Chem. Abstr. 1994, 121, 205336. [Google Scholar]
- Fhamy, H.T. Synthesis and antimicrobial screening of some novel thiazoles, dithiazoles and thiazolylpyridines. Pharmazie 1997, 52, 750–753. [Google Scholar]
- Pandeya, S.N.; Sriram, D.; Nath, G.; Declercq, E. Synthesis, antibacterial, antifungal and anti- HIV activities of Schiff and Mannich bases derived from isatin derivatives and N-[4,(4’- Chlorophenyl)thiazol-2-yl]thiosemicarbazide. Eur. J. Pharm. Sci. 1999, 9, 25–31. [Google Scholar] [CrossRef]
- Ateş, Ö.; Altintas, H.; Ötük, G. Synthesis and antimicrobial activity of 4-Carbethoxymethyl-2- [(α-haloacyl)amino]thiazoles and 5-non-substituted/substituted 2-[(4-Carbethoxymethylthiazol-2- yl)imino]-4-thiazolidinones. Arzneimittelforschung 2000, 50, 569–575. [Google Scholar] [PubMed]
- Lakhan, R.; Sharma, B.P.; Shukla, B.N. Synthesis and antimicrobial activity of 1-aryl-2-amino-3-(4-arylthiazol-2-yl)/(benzothiazol-2-yl)guanidines. Farmaco 2000, 55, 331–337. [Google Scholar] [CrossRef]
- Kaplancikli, Z.A.; Turan-Zitouni, G.; Revial, G.; Güven, K. Synthesis and study of antibacterial and antifungal activities of novel 2-[[(benzoxazole/benzimidazole-2-yl)sulfanyl]acetylamino] thiazoles. Arch. Pharm. Res. 2004, 27, 1081–1085. [Google Scholar] [CrossRef] [PubMed]
- Turan-Zitouni, G.; Demirayak, Ş.; Özdemir, A.; Kaplancıklı, Z.A.; Yıldız, M.T. Synthesis of some 2-[(benzazole-2-yl)thioacetylamino]thiazole derivatives and their antimicrobial activity and toxicity. Eur. J. Med. Chem. 2004, 39, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Ashtekar, D.R.; Fernandes, F.; Khadse, B.G.; Shirodkar, M.V.A. A rapid method for the evaluation of new antituberculosis agents. Chemotherapy 1987, 33, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Maass, G.; Immendoerfer, U.; Koenig, B.; Leser, U.; Mueller, B.; Goody, R.; Pfatt, B. Viral resistance to the thiazolo- iso- indolinones, a new class of nonnucleoside inhibitors of HIV virus type 1 reverse transcriptase. Antimicrob. Agents Chemother. 1993, 37, 2612–2617. [Google Scholar] [CrossRef] [PubMed]
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Jothikrishnan, B.T.; Narasimhan, S.; Shafi, S.S. 2-{5-(1,3-Benzodioxol-5-yl)-1-[4-(4-chlorophenyl)-1,3-thiazol-2-yl]-4,5-dihydro-1H-pyrazol-3-yl}pyrazine. Molbank 2010, 2010, M668. https://doi.org/10.3390/M668
Jothikrishnan BT, Narasimhan S, Shafi SS. 2-{5-(1,3-Benzodioxol-5-yl)-1-[4-(4-chlorophenyl)-1,3-thiazol-2-yl]-4,5-dihydro-1H-pyrazol-3-yl}pyrazine. Molbank. 2010; 2010(1):M668. https://doi.org/10.3390/M668
Chicago/Turabian StyleJothikrishnan, Balapragalathan Thappali, Sriram Narasimhan, and Suban Syed Shafi. 2010. "2-{5-(1,3-Benzodioxol-5-yl)-1-[4-(4-chlorophenyl)-1,3-thiazol-2-yl]-4,5-dihydro-1H-pyrazol-3-yl}pyrazine" Molbank 2010, no. 1: M668. https://doi.org/10.3390/M668
APA StyleJothikrishnan, B. T., Narasimhan, S., & Shafi, S. S. (2010). 2-{5-(1,3-Benzodioxol-5-yl)-1-[4-(4-chlorophenyl)-1,3-thiazol-2-yl]-4,5-dihydro-1H-pyrazol-3-yl}pyrazine. Molbank, 2010(1), M668. https://doi.org/10.3390/M668