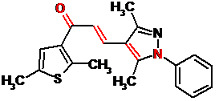
(2E)-3-(3,5-Dimethyl-1-phenyl-1H-pyrazol-4-yl)-1-(2,5-dimethyl-3-thienyl)prop-2-en-1-one
Abstract
:Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgements
References and Notes
- Batovska, D.; Parushev, S.; Stamboliyska, B.; Tsvetkova, I.; Ninova, M.; Najdenski, H. Examination of growth inhibitory properties of synthetic chalcones for which antibacterial activity was predicted. Eur. J. Med. Chem. 2009, 44, 2211–2218. [Google Scholar] [CrossRef] [PubMed]
- Bandgar, B.P.; Gawande, S.S. Synthesis and biological screening of a combinatorial library of β-chlorovinyl chalcones as anticancer, anti-inflammatory and antimicrobial agents. Bior. Med. Chem. 2010, 18, 2060–2065. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.F.; Boesen, T.; Larsen, M.; Schonning, K.; Kromann, H. Antibacterial chalcones –bioisosteric replacement of the 4′-hydroxy group. Bioorg. Med. Chem. 2004, 12, 3047–3054. [Google Scholar] [CrossRef] [PubMed]
- Ozdemir, Z.; Kandilci, H.B.; Gumusel, B.; Calıs, U.; Bilgin, A.A. Synthesis and studies on antidepressant and anticonvulsant activities of some 3-(2-furyl)-pyrazoline derivatives. Eur. J. Med. Chem. 2007, 42, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Ansari, F.L.; Iftikhar, F.; Ihsan-ul-Haq; Mirza, B.; Baseer, M.; Rashid, U. Solid-phase synthesis and biological evaluation of a parallel library of 2,3-dihydro-1,5-benzothiazepines. Bioorg. Med. Chem. 2008, 16, 7691–7697. [Google Scholar] [CrossRef] [PubMed]
- Asiri, A.M.; Khan, S.A. (2E,2'E)-3,3-(1,4-Phenylene)bis[1-(2,5-dimethyl-3-thienyl)prop-2-en-1-one]. Molbank 2009, 2009, M636. [Google Scholar] [CrossRef]
- Holla, B.S.; Akberali, P.M.; Shivananda, M.K. Studies on arylfuran derivatives: Part X. Synthesis and antibacterial properties of arylfuryl-Δ2-pyrazolines. Il Farmaco 2000, 55, 256–263. [Google Scholar] [PubMed]
- Chimenti, F.; Bizzarri, B.; Manna, F.; Bolasco, A.; Secci, D.; Chimenti, P.; Granese, A.; Rivanera, D.; Lilli, D.; Scaltrito, M.M.; Brenciaglia, M.I. Synthesis and in vitro selective anti-Helicobacter pylori activity of pyrazoline derivatives. Bioorg. Med. Chem. Lett. 2005, 15, 603–607. [Google Scholar] [CrossRef] [PubMed]
- Manna, F.; Chimenti, F.; Bolasco, A.; Cenicola, M.L.; Amico, M.D.; Parrillo, C.; Rossi, F.; Marmo, E. Anti-inflammatory, analgesic and antipyretic N-acetyl-Δ2-pyrazolines and dihydro-thienocoumarines. Eur. J. Med. Chem. 1992, 27, 633–639. [Google Scholar] [CrossRef]

© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Asiri, A.M.; Khan, S.A. (2E)-3-(3,5-Dimethyl-1-phenyl-1H-pyrazol-4-yl)-1-(2,5-dimethyl-3-thienyl)prop-2-en-1-one. Molbank 2010, 2010, M679. https://doi.org/10.3390/M679
Asiri AM, Khan SA. (2E)-3-(3,5-Dimethyl-1-phenyl-1H-pyrazol-4-yl)-1-(2,5-dimethyl-3-thienyl)prop-2-en-1-one. Molbank. 2010; 2010(2):M679. https://doi.org/10.3390/M679
Chicago/Turabian StyleAsiri, Abdullah M., and Salman A. Khan. 2010. "(2E)-3-(3,5-Dimethyl-1-phenyl-1H-pyrazol-4-yl)-1-(2,5-dimethyl-3-thienyl)prop-2-en-1-one" Molbank 2010, no. 2: M679. https://doi.org/10.3390/M679
APA StyleAsiri, A. M., & Khan, S. A. (2010). (2E)-3-(3,5-Dimethyl-1-phenyl-1H-pyrazol-4-yl)-1-(2,5-dimethyl-3-thienyl)prop-2-en-1-one. Molbank, 2010(2), M679. https://doi.org/10.3390/M679