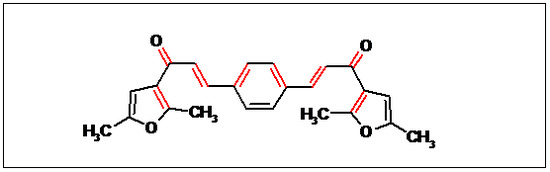
2E,2'E-3,3'-(1,4-Phenylene)bis(1-(2,5-dimethylfuran-3-yl)prop-2-en-1-one
Abstract
:Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgements
References
- Vogel, S.; Barbic, M.; Jurgenliemk, G.; Heilmann, J. Synthesis, cytotoxicity, anti-oxidative and anti-inflammatory activity of chalcones and influence of A-ring modifications on the pharmacological effect. Eur. J. Med. Chem. 2010, 45, 2206–2213. [Google Scholar] [CrossRef] [PubMed]
- Kamal, A.; Balakishan, G.; Ramakrishna, G.; Shaik, T.B.; Sreekanth, K.; Balakrishna, M.; Rajender, D.; Kalivendi, S.V. Synthesis and biological evaluation of cinnamido linked pyrrolo[2,1-c][1,4]benzodiazepines as antimitotic agents. Eur. J. Med. Chem. 2010, 45, 3870–3884. [Google Scholar] [CrossRef] [PubMed]
- Reichwald, C.; Shimony, O.; Sacerdoti-Sierra, N.; Jaffe, C.L.; Kunick, C. A new Heck reaction modification using ketone Mannich bases as enone precursors: Parallel synthesis of anti-leishmanial chalcones. Bioorg. Med. Chem. Lett. 2008, 18, 1985–1989. [Google Scholar] [CrossRef] [PubMed]
- Katritzky, A.R.; Kuanar, M.; Dobchev, D.A.; Vanhoecke, B.W.A.; Karelson, M.; Parmar, V.S.; Stevens, C.V.; Bracke, M.E. QSAR modeling of anti-invasive activity of organic compounds using structural descriptors. Bioorg. Med. Chem. 2006, 14, 6933–6939. [Google Scholar] [CrossRef] [PubMed]
- Hussain, T.; Siddiqui, H.L.; Zia-ur-Rehman, M.; Yasinzai, M.M.; Parvez, M. Anti-oxidant, anti-fungal and anti-leishmanial activities of novel 3-[4-(1H-imidazol-1-yl) phenyl]prop-2-en-1-ones. Eur. J. Med. Chem. 2009, 44, 4654–4660. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wilairat, P.; Go, M. Antimalarial activity of ferrocenyl chalcones. Bioorg. Med. Chem. Lett. 2002, 12, 2299–2302. [Google Scholar] [CrossRef]
- Rao, Y.K.; Fang, S.; Tzeng, Y. Synthesis, growth inhibition, and cell cycle evaluations of novel flavonoid derivatives. Bioorg. Med. Chem. 2005, 13, 6850–6855. [Google Scholar] [CrossRef] [PubMed]

© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Asiri, A.M.; Khan, S.A. 2E,2'E-3,3'-(1,4-Phenylene)bis(1-(2,5-dimethylfuran-3-yl)prop-2-en-1-one. Molbank 2010, 2010, M694. https://doi.org/10.3390/M694
Asiri AM, Khan SA. 2E,2'E-3,3'-(1,4-Phenylene)bis(1-(2,5-dimethylfuran-3-yl)prop-2-en-1-one. Molbank. 2010; 2010(3):M694. https://doi.org/10.3390/M694
Chicago/Turabian StyleAsiri, Abdullah M., and Salman A. Khan. 2010. "2E,2'E-3,3'-(1,4-Phenylene)bis(1-(2,5-dimethylfuran-3-yl)prop-2-en-1-one" Molbank 2010, no. 3: M694. https://doi.org/10.3390/M694
APA StyleAsiri, A. M., & Khan, S. A. (2010). 2E,2'E-3,3'-(1,4-Phenylene)bis(1-(2,5-dimethylfuran-3-yl)prop-2-en-1-one. Molbank, 2010(3), M694. https://doi.org/10.3390/M694