Abstract
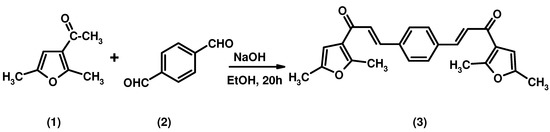
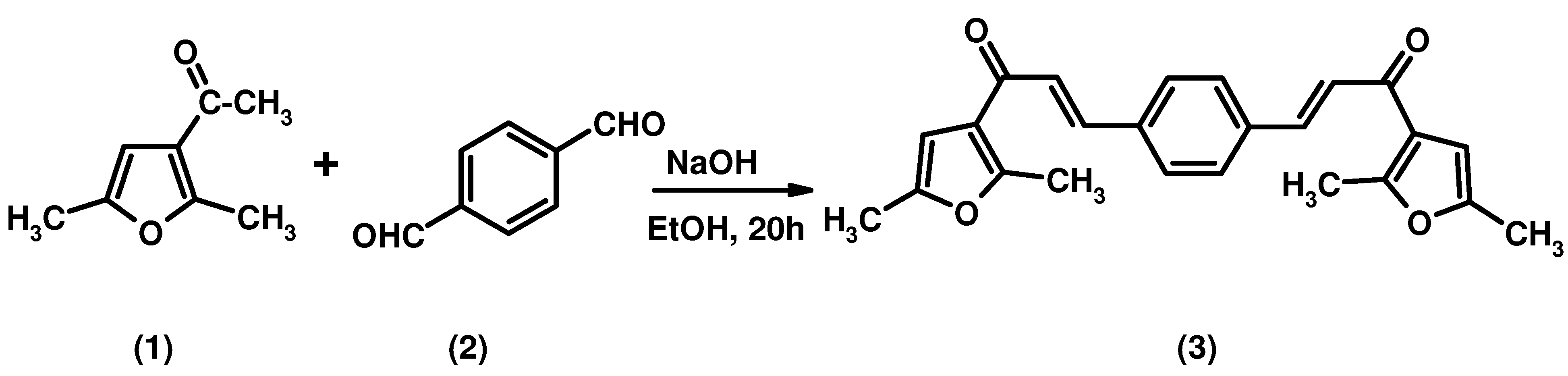
A bis-chalcone has been synthesized by reaction of 3-acetyl-2,5-dimethylfuran and terephthalaldehyde in ethanolic NaOH at room temperature: (2E,2'E)-3,3'-(1,4-phenylene)bis(1-(2,5-dimethylfuran-3-yl)prop-2-en-1-one) (3) was obtained in high yield. The structure of this compound was established by elemental analysis, IR, 1H NMR, 13C NMR and EI-MS spectral analysis.
The Claisen-Schmidt condensation is the most important reaction for the formation of 1,3-diphenyl-2-propene-1-ones. The products, also known as chalcones, generally are synthesized by this method from suitable acetophenones and benzaldehydes. Chalcones are considered to be precursors of flavonoids when found as naturally-occurring compounds. The chemical importance of chalcones is extended in two branches: their biological activity, including anti-inflammatory [1], antimitotic [2], anti-leishmanial [3], anti-invasive [4], anti fungal [5], antimalarial [6] and anti-tumor [7] properties; as well as their recognized synthetic utility in the preparation of pharmacologically interesting heterocyclic systems such as thiazines, pyrimidines, and pyrazoles. Here we are reporting a novel bis-chalcone prepared from 3-acetyl-2,5-dimethylfuran and terephthalaldehyde. The product is assumed to exist as one E,E-diastereomer, since in the the 1H-NMR spectrum the olefinic protons display coupling constants of 15.6 Hz indicative of the E-configuration.
A solution of 3-acetyl-2,5-dimethylfuran (2.34 mL, 0.028 mol) and terephthalaldehyde (2.0 g, 0.014 mol) in an ethanolic solution of NaOH (6.0 g in 10 mL of ethanol) was stirred for 20 h at room temperature. The solution was poured into ice cold water of pH~2 (pH adjusted by HCl). The solid was separated and dissolved in CH2Cl2, washed with a saturated solution of NaHCO3 and evaporated to dryness. The residue was recrystallized from methanol/chloroform to give a yellow solid:
Yield: 78%; m.p. 182 °C.
EI-MS m/z (rel. int.%): 376 (76) [M+1]+,
IR (KBr) vmax cm−1: 2956 (C-H), 1655 (C=O), 1567 (C=C).
1H NMR (600 MHz, CDCl3) δ: 7.72 (d, 2H, J = 15.6 Hz, C=CH), 7.22 (d, 2H, J = 15.6 Hz, CO=CH), 7.63 (s, 4H, Ar-H), 6.34 (s, 2H, 2×furan-H), 2.62 (s, 6H, 2×CH3), 2.30 (s, 6H, 2×CH3).
13CNMR (150 MHz, CDCl3) δ: 185.65, 158.20, 150.17, 141.60, 136.70, 128.99, 125.00, 122.41, 105.54, 14.53, 13.05.
Anal. calc. for C24H22O4: C, 76.99, H, 5.92, O, 17.09; Found: C, 76.95, H, 5.88, O, 19.98.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgements
The authors would like to thank the deanship of scientific research for the financial support of this work via Grant No. (3-045/430).
References
- Vogel, S.; Barbic, M.; Jurgenliemk, G.; Heilmann, J. Synthesis, cytotoxicity, anti-oxidative and anti-inflammatory activity of chalcones and influence of A-ring modifications on the pharmacological effect. Eur. J. Med. Chem. 2010, 45, 2206–2213. [Google Scholar] [CrossRef] [PubMed]
- Kamal, A.; Balakishan, G.; Ramakrishna, G.; Shaik, T.B.; Sreekanth, K.; Balakrishna, M.; Rajender, D.; Kalivendi, S.V. Synthesis and biological evaluation of cinnamido linked pyrrolo[2,1-c][1,4]benzodiazepines as antimitotic agents. Eur. J. Med. Chem. 2010, 45, 3870–3884. [Google Scholar] [CrossRef] [PubMed]
- Reichwald, C.; Shimony, O.; Sacerdoti-Sierra, N.; Jaffe, C.L.; Kunick, C. A new Heck reaction modification using ketone Mannich bases as enone precursors: Parallel synthesis of anti-leishmanial chalcones. Bioorg. Med. Chem. Lett. 2008, 18, 1985–1989. [Google Scholar] [CrossRef] [PubMed]
- Katritzky, A.R.; Kuanar, M.; Dobchev, D.A.; Vanhoecke, B.W.A.; Karelson, M.; Parmar, V.S.; Stevens, C.V.; Bracke, M.E. QSAR modeling of anti-invasive activity of organic compounds using structural descriptors. Bioorg. Med. Chem. 2006, 14, 6933–6939. [Google Scholar] [CrossRef] [PubMed]
- Hussain, T.; Siddiqui, H.L.; Zia-ur-Rehman, M.; Yasinzai, M.M.; Parvez, M. Anti-oxidant, anti-fungal and anti-leishmanial activities of novel 3-[4-(1H-imidazol-1-yl) phenyl]prop-2-en-1-ones. Eur. J. Med. Chem. 2009, 44, 4654–4660. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wilairat, P.; Go, M. Antimalarial activity of ferrocenyl chalcones. Bioorg. Med. Chem. Lett. 2002, 12, 2299–2302. [Google Scholar] [CrossRef]
- Rao, Y.K.; Fang, S.; Tzeng, Y. Synthesis, growth inhibition, and cell cycle evaluations of novel flavonoid derivatives. Bioorg. Med. Chem. 2005, 13, 6850–6855. [Google Scholar] [CrossRef] [PubMed]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).