(E)-1-(2-Hydroxy-4,6-dimethoxyphenyl)-3-[4-methoxy-3-(3-methylbut-2-en-1-yl)phenyl]prop-2-en-1-one
Abstract
:1. Introduction

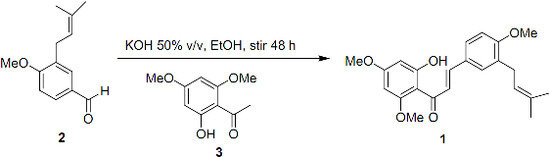
2. Synthesis
3. Experimental
3.1. (E)-1-(2-Hydroxy-4,6-dimethoxyphenyl)-3-[4-methoxy-3-(3-methylbut-2-en-1-yl)phenyl]prop-2-en-1-one (1)
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgements
References
- Narender, T.; Shweta, K.; Tanvir, M.; Rao, M.S.; Srivastava, K.; Puri, S.K. Prenylated chalcones isolated from Crotalaria genus inhibits in vitro growth of the human malaria parasite Plasmodium falciparum. Bioorg. Med. Chem. Lett. 2005, 15, 2453–2455. [Google Scholar] [CrossRef] [PubMed]
- Enoki, T.; Ohnogi, H.; Nagamine, K.; Kudo, Y.; Sugiyama, K.; Tanabe, M.; Kobayashi, E.; Sagawa, H.; Kato, I. Antidiabetic Activities of Chalcones Isolated from a Japanese Herb. Angelica keiskei. J. Agric. Food Chem. 2007, 55, 6013–6017. [Google Scholar] [CrossRef] [PubMed]
- Jayasinghe, L.; Balasooriya, B.A.I.S.; Padmini, W.C.; Hara, N.; Fujimoto, Y. Geranyl chalcone derivatives with antifungal and radical scavenging properties from the leaves of Artocarpus nobilis. Phytochemistry 2004, 65, 1287–1290. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.; Fan, C.Q.; Wang, Y.; Dong, L.; Yue, J.M. Antibacterial prenylflavone derivatives from Psoralea corylifolia, and their structure-activity relationship study. Bioorg. Med. Chem. Lett. 2004, 12, 4387–4392. [Google Scholar] [CrossRef] [PubMed]
- Tabata, K.; Motani, K.; Takayanagi, N.; Nishimura, R.; Asami, S.; Kimura, Y.; Ukiya, M.; Hasegawa, D.; Akihisa, T.; Suzuki, T. Xanthoangelol, a Major Chalcone Constituent of Angelica keiskei, Induces Apoptosis in Neuroblastoma and Leukemia Cells. Biol. Pharm. Bull. 2005, 28, 1404–1407. [Google Scholar] [CrossRef] [PubMed]
- Haraguchi, H.; Ishikawa, H.; Mizutani, K.; Tamura, Y.; Kinoshita, T. Antioxidative and superoxide scavenging activities of retrochalcones in Glycyrrhiza inflata. Bioorg. Med. Chem. 1998, 6, 339–347. [Google Scholar] [CrossRef]
- Hsieh, H.K.; Lee, T.H.; Wang, J.P.; Wang, J.J.; Lin, C.N. Synthesis and Anti-inflammatory Effect of Chalcones and Related Compounds. Pharm. Res. 1998, 15, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Barron, D.; Balland, C.; Possety, F.; Ravanel, P.; Desfougeres, A. Flavonoïdes prénylés et perméabilité membranaire. Acta Bot. Gall. 1996, 143, 509–520. [Google Scholar] [CrossRef]
- Adib, A.M.; Ahmad, F.; Idris, M.S. Synthesis and Antimicrobial Activity of 4’,5,7-Trihydroxy-3’-prenylflavanone. J. Chem. Sci. 2008, 120, 469–473. [Google Scholar] [CrossRef] [Green Version]
- Rao, G.V.; Swamy, B.N.; Chandregowda, V.; Reddy, G.C. Synthesis of (±) Abyssinone I and related compounds: Their anti-oxidant and cytotoxic activities. Eur. J. Med. Chem. 2009, 44, 2239–2245. [Google Scholar] [CrossRef] [PubMed]
- Reddy, N.P.; Aparoy, P.; Reddy, C.M.; Achari, C.; Sridhar, P.R.; Reddanna, P. Design, synthesis and biological evaluation of prenylated chalcones as 5-LOX inhibitors. Biorg. Med. Chem. 2010, 18, 5807–5815. [Google Scholar] [CrossRef] [PubMed]
© 2010 by the author; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Adib, A.M. (E)-1-(2-Hydroxy-4,6-dimethoxyphenyl)-3-[4-methoxy-3-(3-methylbut-2-en-1-yl)phenyl]prop-2-en-1-one. Molbank 2010, 2010, M708. https://doi.org/10.3390/M708
Adib AM. (E)-1-(2-Hydroxy-4,6-dimethoxyphenyl)-3-[4-methoxy-3-(3-methylbut-2-en-1-yl)phenyl]prop-2-en-1-one. Molbank. 2010; 2010(4):M708. https://doi.org/10.3390/M708
Chicago/Turabian StyleAdib, Adiana Mohamed. 2010. "(E)-1-(2-Hydroxy-4,6-dimethoxyphenyl)-3-[4-methoxy-3-(3-methylbut-2-en-1-yl)phenyl]prop-2-en-1-one" Molbank 2010, no. 4: M708. https://doi.org/10.3390/M708
APA StyleAdib, A. M. (2010). (E)-1-(2-Hydroxy-4,6-dimethoxyphenyl)-3-[4-methoxy-3-(3-methylbut-2-en-1-yl)phenyl]prop-2-en-1-one. Molbank, 2010(4), M708. https://doi.org/10.3390/M708