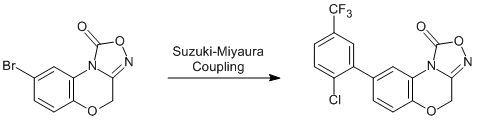
8-[2-Chloro-5-(trifluoromethyl)phenyl]-4H-[1,2,4]oxadiazolo-[3,4-c][1,4]benzoxazin-1-one
Abstract
Share and Cite
Berezin, A.A.; Koutentis, P.A. 8-[2-Chloro-5-(trifluoromethyl)phenyl]-4H-[1,2,4]oxadiazolo-[3,4-c][1,4]benzoxazin-1-one. Molbank 2011, 2011, M728. https://doi.org/10.3390/M728
Berezin AA, Koutentis PA. 8-[2-Chloro-5-(trifluoromethyl)phenyl]-4H-[1,2,4]oxadiazolo-[3,4-c][1,4]benzoxazin-1-one. Molbank. 2011; 2011(2):M728. https://doi.org/10.3390/M728
Chicago/Turabian StyleBerezin, Andrey A., and Panayiotis A. Koutentis. 2011. "8-[2-Chloro-5-(trifluoromethyl)phenyl]-4H-[1,2,4]oxadiazolo-[3,4-c][1,4]benzoxazin-1-one" Molbank 2011, no. 2: M728. https://doi.org/10.3390/M728
APA StyleBerezin, A. A., & Koutentis, P. A. (2011). 8-[2-Chloro-5-(trifluoromethyl)phenyl]-4H-[1,2,4]oxadiazolo-[3,4-c][1,4]benzoxazin-1-one. Molbank, 2011(2), M728. https://doi.org/10.3390/M728