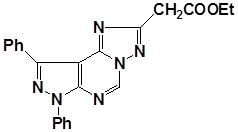
Ethyl (1,3-diphenyl-1H-pyrazolo[4,3-e][1,2,4]triazolo[1,5-c]pyrimidin-5-yl)acetate
Abstract
:Results and Discussion
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3References and Notes
- Nagamatsu, T.; Fujita, T. Facile and general syntheses of 3- and/or 5-substituted 7H-pyrazolo[4,3- e]-1,2,4-triazolo[4,3-c]pyrimidines as a new class of potential xanthine oxidase inhibitors. Chem. Commun. 1999, 1461–1462. [Google Scholar] [CrossRef]
- Ongini, E.; Monopoli, A.; Cacciari, B.; Baraldi, P.G. Selective adenosine A2A receptor antagonists. Il Farmaco 2001, 56, 87–90. [Google Scholar] [CrossRef]
- Kishore, D.P.; Balakumar, C.; Rao, A.R.; Roy, P.P.; Roy, K. QSAR of adenosine receptor antagonists: Exploring physicochemical requirements for binding of pyrazolo[4,3-e]-1,2,4-triazolo[1,5-c]pyrimidine derivatives with human adenosine A3 receptor subtype. Bioorg. Med. Chem. Lett. 2011, 21, 818–823. [Google Scholar] [CrossRef] [PubMed]
- Baraldi, P.G.; Manfredini, S.; Simoni, D.; Zappaterra, L.; Zocchi, C.; Dionisotti, S.; Ongini, E. Synthesis of new pyrazolo[4,3-e]1,2,4-triazolo[1,5-c]pyrimidine and 1,2,3-triazolo[4,5-e]1,2,4-triazolo[1,5-c]pyrimidine displaying potent and selective activity as A2a adenosine receptor antagonists. Bioorg. Med. Chem. Lett. 1994, 4, 2539–2544. [Google Scholar] [CrossRef]
- Kumar, T.S.; Mishra, S.; Deflorian, F.; Yoo, L.S.; Phan, K.; Kecskés, M.; Szabo, A.; Shinkre, B.; Gao, Z.-G.; Trenkle, W.; Jacobson, K.A. Molecular probes for the A2A adenosine receptor based on a pyrazolo[4,3-e][1,2,4]triazolo[1,5-c]pyrimidin-5-amine scaffold. Bioorg. Med. Chem. Lett. 2011, 21, 2740–2745. [Google Scholar] [CrossRef] [PubMed]
- Dolzhenko, A.V.; Pastorin, G.; Dolzhenko, A.V.; Chui, W.K. A new synthesis of 2,8-disubstituted pyrazolo[4,3-e][1,2,4]triazolo[1,5-c]pyrimidines. Tetrahedron Lett. 2009, 50, 5617–5621. [Google Scholar] [CrossRef]
- Baraldi, P.G.; El-Kashef, H.; Farghaly, A.; Vanelle, P.; Fruttarolo, F. A new synthesis of 2,8-disubstituted pyrazolo[4,3-e][1,2,4]triazolo[1,5-c]pyrimidines and related heterocycles. Tetrahedron 2004, 60, 5093–5104. [Google Scholar] [CrossRef]
- Harris, J.M.; Neustadt, B.R.; Zhang, H.; Lachowicz, J.; Cohen-Williams, M.; Varty, G.; Hao, J.; Stamford, A.W. Potent and selective adenosine A2A receptor antagonists: [1,2,4]Triazolo[4,3-c]pyrimidin-3-ones. Bioorg. Med. Chem. Lett. 2011, 21, 2497–2501. [Google Scholar] [CrossRef] [PubMed]
- Baraldi, P.G.; Bovero, A.; Fruttarolo, F.; Romagnoli, R.; Tabrizi, M.A.; Preti, D.; Varani, K.; Borea, P.A.; Moorman, A.R. New strategies for the synthesis of A3 adenosine receptor antagonists. Bioorg. Med. Chem. 2003, 11, 4161–4169. [Google Scholar] [CrossRef]
- Michielan, L.; Bolcato, C.; Federico, S.; Cacciari, B.; Bacilieri, M.; Klotz, K.-N.; Kachler, S.; Pastorin, G.; Cardin, R.; Sperduti, A.; Spalluto, G.; Moro, S. Combining selectivity and affinity predictions using an integrated Support Vector Machine (SVM) approach: An alternative tool to discriminate between the human adenosine A2A and A3 receptor pyrazolo-triazolo-pyrimidine antagonists binding sites. Bioorg. Med. Chem. 2009, 17, 5259–5274. [Google Scholar] [CrossRef] [PubMed]
- Paeshuyse, J.; Letellier, C.; Froeyen, M.; Dutartre, H.; Vrancken, R.; Canard, B.; De Clercq, E.; Gueiffier, A.; Teulade, J.-C.; Herdewijn, P.; Puerstinger, G.; Koenen, F.; Kerkhofs, P.; Baraldi, P.G.; Neyts, J. A pyrazolotriazolopyrimidinamine inhibitor of bovine viral diarrhea virus replication that targets the viral RNA-dependent RNA polymerase. Antiviral Res. 2009, 82, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Shawali, A.S.; Fahmi, A.A.; Albar, H.A.; Hassaneen, H.M.; Abdelhamid, H.A. A facile synthesis of some pyrazolo analogues of tricyclic purine derivatives via hydrazonoyl halides. J. Chem. Res. (S) 1994, 6, 1040–1049. [Google Scholar]

© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Farghaly, T.A.; Gomha, S.M. Ethyl (1,3-diphenyl-1H-pyrazolo[4,3-e][1,2,4]triazolo[1,5-c]pyrimidin-5-yl)acetate. Molbank 2011, 2011, M743. https://doi.org/10.3390/M743
Farghaly TA, Gomha SM. Ethyl (1,3-diphenyl-1H-pyrazolo[4,3-e][1,2,4]triazolo[1,5-c]pyrimidin-5-yl)acetate. Molbank. 2011; 2011(4):M743. https://doi.org/10.3390/M743
Chicago/Turabian StyleFarghaly, Thoraya A., and Sobhi M. Gomha. 2011. "Ethyl (1,3-diphenyl-1H-pyrazolo[4,3-e][1,2,4]triazolo[1,5-c]pyrimidin-5-yl)acetate" Molbank 2011, no. 4: M743. https://doi.org/10.3390/M743
APA StyleFarghaly, T. A., & Gomha, S. M. (2011). Ethyl (1,3-diphenyl-1H-pyrazolo[4,3-e][1,2,4]triazolo[1,5-c]pyrimidin-5-yl)acetate. Molbank, 2011(4), M743. https://doi.org/10.3390/M743