2-[(1-Benzamido-2-methoxy-2-oxoethyl)amino]benzoic Acid
Abstract
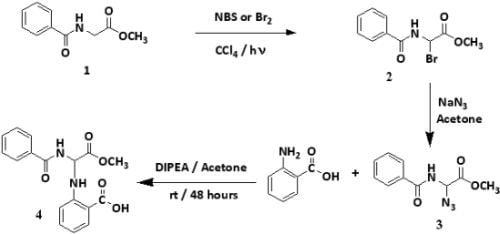
:1. Introduction
2. Results and Discussion
3. Experimental
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3References and Notes
- Beller, M.; Eckert, M. Amidocarbonylation—An efficient route to amino acid derivatives. Angew. Chem. Int. Ed. 2000, 39, 1011–1027. [Google Scholar] [CrossRef]
- Samel, M.; Tõnismägi, K.; Rönnholm, G.; Vija, H.; Siigur, J.; Kalkkinen, N.; Siigur, E. l-Amino acid oxidase from Naja naja oxiana venom. Comp. Biochem. Physiol. B 2008, 149, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Eddy, N.O. Experimental and theoretical studies on some amino acids and their potential activity as inhibitors for the corrosion of mild steel, part 2. J. Adv. Res. 2011, 2, 35–47. [Google Scholar] [CrossRef]
- Sant’Ana, C.D.; Menaldo, D.L.; Costa, T.R.; Godoy, H.; Muller, V.D.M.; Aquino, V.H.; Albuquerque, S.; Sampaio, S.V.; Monteiro, M.C.; Stábeli, R.G.; et al. Antiviral and antiparasite properties of an L-amino acid oxidase from the snake Bothrops jararaca: Cloning and identification of a complete cDNA sequence. Biochem. Pharmacol. 2008, 76, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Stábeli, R.G.; Sant’Ana, C.D.; Ribeiro, P.H.; Costa, T.R.; Ticli, F.K.; Pires, M.G.; Nomizob, A.; Albuquerque, S.; Malta-Neto, N.R.; Marins, M.; et al. Cytotoxic l-amino acid oxidase from Bothrops moojeni: Biochemical and functional characterization. Int. J. Biol. Macromol. 2007, 41, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Samel, M.; Vija, H.; Rönnholm, G.; Siigur, J.; Kalkkinen, N.; Siigur, E. Isolation and characterization of an apoptotic and platelet aggregation inhibiting L-amino acid oxidase from Vipera berus berus (common viper) venom. Biochim. Biophys. Acta 2006, 1764, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, P.; Corey, E.J. A short, stereocontrolled, and practical synthesis of alpha-methylomuralide, a potent inhibitor of proteasome function. J. Org. Chem. 2003, 68, 2760–2764. [Google Scholar] [CrossRef] [PubMed]
- Mabrouk, E.H.; Abdelrhani, E.; Abdelilah, E.H.; Anouar, A.; Soumia, E.H.; Jean, M.; Vallery, R. Synthesis of new racemic α-heterocyclic α,α-diaminoesters and α-aminoester carboxylic. Arab. J. Chem. 2013, 6, 93–96. [Google Scholar] [CrossRef]
- Kober, R.; Steglich, W. Untersuchungen zur Reaktion von Acylaminobrommalonestern und Acylaminobromessigestern mit Trialkylphosphiten-eine einfache Synthese von 2-Amino-2-(diethoxyphosphoryl) Essigsäure Ethylester. Liebigs Ann. Chem. 1983, 1983, 599–609. [Google Scholar] [CrossRef]
- Achamlale, S.; Elachqar, A.; El Hallaoui, A.; El Hajji, S.; Roumestant, M.L.; Viallefont, P.H. Synthesis of α-triazolyl α-aminoacid derivatives. Amino Acids 1997, 12, 257–263. [Google Scholar] [CrossRef]

© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
El Houssine, M.; Abdelrhani, E.; Abdelilah, E.H.; Anouar, A. 2-[(1-Benzamido-2-methoxy-2-oxoethyl)amino]benzoic Acid. Molbank 2013, 2013, M792. https://doi.org/10.3390/M792
El Houssine M, Abdelrhani E, Abdelilah EH, Anouar A. 2-[(1-Benzamido-2-methoxy-2-oxoethyl)amino]benzoic Acid. Molbank. 2013; 2013(1):M792. https://doi.org/10.3390/M792
Chicago/Turabian StyleEl Houssine, Mabrouk, Elachqar Abdelrhani, El Hallaoui Abdelilah, and Alami Anouar. 2013. "2-[(1-Benzamido-2-methoxy-2-oxoethyl)amino]benzoic Acid" Molbank 2013, no. 1: M792. https://doi.org/10.3390/M792
APA StyleEl Houssine, M., Abdelrhani, E., Abdelilah, E. H., & Anouar, A. (2013). 2-[(1-Benzamido-2-methoxy-2-oxoethyl)amino]benzoic Acid. Molbank, 2013(1), M792. https://doi.org/10.3390/M792