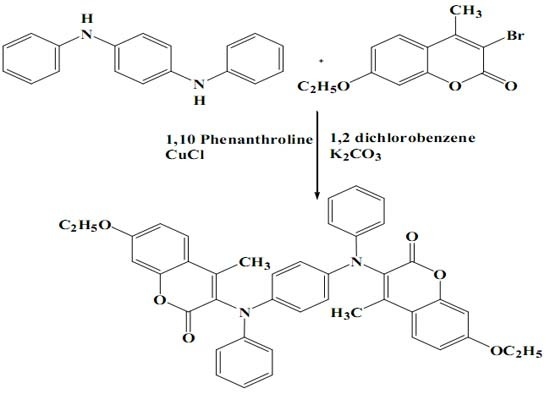
3,3'-(1,4-Phenylenebis(phenylazanediyl))bis(7-ethoxy-4-methyl-2H-chromen-2-one)
Abstract
:Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgements
References
- Chen, C.T.; Chiang, C.L.; Lin, Y.C.; Chan, L.H.; Huang, C.H.; Tsai, Z.W. Synthesis and photoluminescent properties of 7-N,N-diphenylamino-3-benzoheterocyclic coumarin derivatives. Org. Lett. 2003, 5, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.W.; VanSlyke, S.A.; Chen, C.H. Electroluminescence of doped organic thin films. J. Appl. Phys. 1989, 65, 3610–3616. [Google Scholar] [CrossRef]
- Lee, M.T.; Yen, C.K.; Yang, W.P.; Chen, H.H.; Liao, C.H.; Tsai, C.H.; Chen, C.H. Efficient Green Coumarin Dopants for Organic Light-Emitting Devices. Org. Lett. 2004, 6, 1241–1246. [Google Scholar] [CrossRef] [PubMed]
- Thelakkat, M.; Schmitz, C.; Hohle, C.; Strohriegl, P.; Schmidt, H.-W.; Hofmann, U.; Schloter, S.; Haarer, D. Novel functional materials based on triarylamines-synthesis and application in electroluminescent devices and photorefractive systems. Phys. Chem. Chem. Phys. 1999, 1, 1693–1698. [Google Scholar] [CrossRef]
- Getautis, V.; Paliulis, O.; Gaidelis, V.; Jankauskas, J.; Sidaravičius, J. Hole-drift mobility in phenylenediamine derivatives possessing methyl substituents. J. Photochem. Photobiol. A Chem. 2002, 151, 39–43. [Google Scholar] [CrossRef]
- Nofal, Z.M.; El-Zahar, M.I.; Abd El-Karim, S.S. Novel coumarin derivatives with expected biological activity. Molecules 2000, 5, 99–113. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, D.; Liebeskind, L.S. Ambient temperature, Ullmann-like reductive coupling of aryl, heteroaryl, and alkenyl halides. J. Org. Chem. 1997, 62, 2312–2313. [Google Scholar] [CrossRef] [PubMed]

© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Narayanan, D.; Haridas, K.R. 3,3'-(1,4-Phenylenebis(phenylazanediyl))bis(7-ethoxy-4-methyl-2H-chromen-2-one). Molbank 2013, 2013, M799. https://doi.org/10.3390/M799
Narayanan D, Haridas KR. 3,3'-(1,4-Phenylenebis(phenylazanediyl))bis(7-ethoxy-4-methyl-2H-chromen-2-one). Molbank. 2013; 2013(2):M799. https://doi.org/10.3390/M799
Chicago/Turabian StyleNarayanan, Divia, and Karickal Raman Haridas. 2013. "3,3'-(1,4-Phenylenebis(phenylazanediyl))bis(7-ethoxy-4-methyl-2H-chromen-2-one)" Molbank 2013, no. 2: M799. https://doi.org/10.3390/M799
APA StyleNarayanan, D., & Haridas, K. R. (2013). 3,3'-(1,4-Phenylenebis(phenylazanediyl))bis(7-ethoxy-4-methyl-2H-chromen-2-one). Molbank, 2013(2), M799. https://doi.org/10.3390/M799