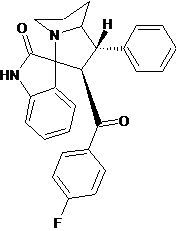
2'-[(4-Fluorophenyl)carbonyl]-1'-phenyl-1',2',5',6',7',7a'-hexahydrospiro[indole-3,3'-pyrrolizin]-2(1H)-one
Abstract
:Introduction
Results and Discussion
Experimental
Reducing Power Assay
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Acknowledgments
References
- Alcaide, B.; Almendros, P.; Alonso, J.M.; Aly, M.F. Rapid and stereocontrolled synthesis of racemic and optically highly functionalized pyrrolidine systems via rearrangement of 1,3-dipolar cycloadducts derived from 2-azetidinone-tethered azomethine ylides. J. Org. Chem. 2001, 66, 1351–1358. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Yang, J.; Gao, S.; He, H.; Li, S.; Di, Y.; Chang, Y.; Lu, Y.; Hao, X. Spiro[pyrrolidine-2,3'-oxindole] derivatives synthesized by novel regionselective 1,3-dipolar cycloadditions. Mol. Divers. 2012, 16, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Rehn, S.; Bergman, J.; Stensland, B. The three-component reaction between isatin, α-amino acids and dipolarophiles. Eur. J. Org. Chem. 2004, 2004, 413–418. [Google Scholar] [CrossRef]
- Prasanna, R.; Purushothaman, S.; Raghunathan, R. Highly regioselective synthesis of glycospiro heterocycles through 1,3-dipolar cycloaddition reaction. Tetrahedron Lett. 2010, 51, 4538–4542. [Google Scholar] [CrossRef]
- Xie, Y.M.; Yao, Y.Q.; Sun, H.B.; Yan, T.T.; Liu, J.; Kang, T.R. Facile synthesis of functionalized spiropyrrolizidine oxindoles via a three-component tandem cycloaddition reaction. Molecules 2011, 16, 8745–8757. [Google Scholar] [CrossRef]
- Dhar, D.N. The Chemistry of Chalcones and Related Compounds; John Wiley: New York, NY, USA, 1981. [Google Scholar]
- Samshuddin, S.; Narayana, B.; Sarojini, B.K.; Yathirajan, H.S.; Raghavendra, R. Synthesis, characterization and biological evaluation of functionalized derivatives of versatile synthon 4,4'-difluoro chalcone. Der Pharma Chemica 2012, 4, 1445–1457. [Google Scholar]
- Jasinski, J.P.; Golen, J.A.; Samshuddin, S.; Narayana, B.; Yathirajan, H.S. Synthesis, characterization and crystal structures of 3,5-bis(4-fluorophenyl)-4,5-dihydro-1H-pyrazole-1-carboxamide and 3,5-bis(4-fluorophenyl)-4,5-dihydro-1H-pyrazole-1-carbothioamide. Crystals 2012, 2, 1108–1115. [Google Scholar] [CrossRef]
- Fun, H.K.; Arshad, S.; Samshuddin, S.; Narayana, B.; Sarojini, B.K. 3,5-Bis(4-fluorophenyl)isoxazole. Acta Cryst. Sect E Struct. Rep. Online 2012, E68, o1783. [Google Scholar] [CrossRef] [PubMed]
- Fun, H.K.; Ooi, C.W.; Sapnakumari, M.; Narayana, B.; Sarojini, B.K. 1-[3-(4 Fluorophenyl)-5-phenyl-4,5-dihydro-1H-pyrazol-1-yl]ethanone. Acta Cryst. Sect. E Struct. Rep. Online 2012, E68, o2634. [Google Scholar] [CrossRef] [PubMed]
- Fun, H.K.; Chia, T.S.; Sapnakumari, M.; Narayana, B.; Sarojini, B.K. 5-(4-Bromophenyl)-3-(4-fluorophenyl)-1-phenyl-4,5-dihydro-1H-pyrazole. Acta Cryst. Sect. E Struct. Rep. Online 2012, E68, o2680. [Google Scholar] [CrossRef] [PubMed]
- Samshuddin, S.; Narayana, B.; Shetty, D.N.; Raghavendra, R. An efficient synthesis of 2,4,6-triaryl pyridines and their biological evaluation. Der Pharma Chemica 2011, 3, 232–240. [Google Scholar]
- Augustine, T.; Prasad, A.; Vithiya, B.S.M.; Ignacimuthu, S. A facile and regioselective synthesis of spiro pyrrolidines and pyrrolizines through 1, 3-dipolar cycloaddition protocol. Der Pharma Chemica 2011, 3, 293–299. [Google Scholar]
- Fokas, D.; Ryan, W.J.; Casebier, D.S.; Coffen, D.L. Solution phase synthesis of a spiro[pyrrolidine-2,3'-oxindole] library via a three component 1,3-dipolar cycloaddition reaction. Tetrahedron Lett. 1998, 39, 2235–2238. [Google Scholar] [CrossRef]
- Chen, G.; He, H.; Ding, J.; Hao, X. Synthesis and antitumor activity evaluation of regioselective spiro[pyrrolidine-2,3'-oxindole] compounds. Heterocycl. Commun. 2009, 15, 355–360. [Google Scholar]
- Vyas, D.H.; Tala, S.D.; Akbari, J.D.; Dhaduk, M.F.; Joshi, K.A.; Joshi, H.S. Synthesis and antimicrobial activity of new cyanopyridine and cyanopyrans towards Mycobacterium Tuberculosis and their microorganisms. Indian J. Chem. 2009, 48B, 833–839. [Google Scholar]
- Fun, H.K.; Loh, W.S.; Sapnakumari, M.; Narayana, B.; Sarojini, B.K. 1-[5-(4-Bromophenyl)-3-(4-fluorophenyl)-4,5-dihydro-1H-pyrazol-1-yl]butan-1-one. Acta Cryst. Sect. E Struct. Rep. Online 2012, E68, o2655–o2656. [Google Scholar] [CrossRef] [PubMed]
- Fun, H.K.; Loh, W.S.; Sapnakumari, M.; Narayana, B.; Sarojini, B.K. 1-[5-(4-Bromophenyl)-3-(4-fluorophenyl)-4,5-dihydro-1H-pyrazol-1-yl]ethanone. Acta Cryst. Sect. E Struct. Rep. Online 2012, E68, o2586. [Google Scholar] [CrossRef] [PubMed]
- Oyaizu, M. Studies on products of the browning reaction. Antioxidative activities of browning reaction products prepared from glucosamine. Jpn. J. Nutr. 1986, 44, 307–315. [Google Scholar] [CrossRef]

© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Sapnakumari, M.; Narayana, B.; Samshuddin, S.; Sarojini, B.K. 2'-[(4-Fluorophenyl)carbonyl]-1'-phenyl-1',2',5',6',7',7a'-hexahydrospiro[indole-3,3'-pyrrolizin]-2(1H)-one. Molbank 2013, 2013, M802. https://doi.org/10.3390/M802
Sapnakumari M, Narayana B, Samshuddin S, Sarojini BK. 2'-[(4-Fluorophenyl)carbonyl]-1'-phenyl-1',2',5',6',7',7a'-hexahydrospiro[indole-3,3'-pyrrolizin]-2(1H)-one. Molbank. 2013; 2013(2):M802. https://doi.org/10.3390/M802
Chicago/Turabian StyleSapnakumari, Majal, Badiadka Narayana, Seranthimata Samshuddin, and Balladka Kunhanna Sarojini. 2013. "2'-[(4-Fluorophenyl)carbonyl]-1'-phenyl-1',2',5',6',7',7a'-hexahydrospiro[indole-3,3'-pyrrolizin]-2(1H)-one" Molbank 2013, no. 2: M802. https://doi.org/10.3390/M802
APA StyleSapnakumari, M., Narayana, B., Samshuddin, S., & Sarojini, B. K. (2013). 2'-[(4-Fluorophenyl)carbonyl]-1'-phenyl-1',2',5',6',7',7a'-hexahydrospiro[indole-3,3'-pyrrolizin]-2(1H)-one. Molbank, 2013(2), M802. https://doi.org/10.3390/M802