5-Pentadecyl-2-((p-tolylimino)methyl)phenol
Abstract
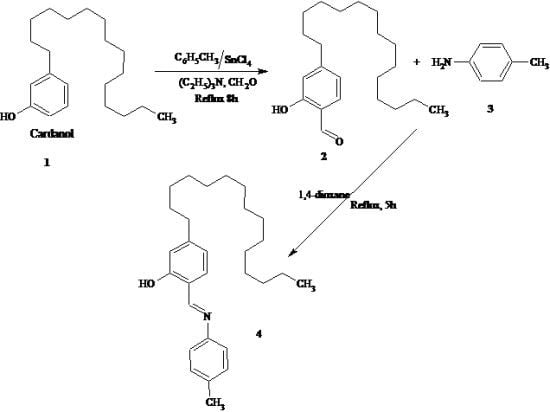
:Introduction
Results
Experimental
Supplementary Materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Acknowledgments
Conflict of Interest
References
- Tarafder, M.T.; Kasbollah, A.; Saravan, N.; Crouse, K.A.; Ali, A.M.; Tin, O.K. Smethyldithiocarbazate and its schiff bases: Evaluation of bondings and biological properties. J. Biochem. Mol. Biol. Biophs. 2002, 6, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Kuçukguzel, Ý.; Kuçukguzel, Þ.G.; Rollas, S.; Otuk-Sanyþ, G.; Ozdemir, O.; Bayrak, Ý.; Altu, T.; Stables, J.P. 3-(Arylalkylthio)-4-alkyl/aryl-5-(4-aminophenyl)-4H-1,2,4-triazole derivatives and their anticonvulsant activity. Farmaco 2004, 59, 893–901. [Google Scholar] [CrossRef] [PubMed]
- Vicini, P.; Geronikaki, A.; Incerti, M.; Busonera, B.; Poni, G.; Kabras, C.A.; Colla, P.L. Synthesis and biological evaluation of benzo[d]isothiazole, benzothiazole and thiazole Schiff bases. Bioorg. Med. Chem. 2003, 11, 4785–4789. [Google Scholar] [CrossRef]
- Kahveci, B.; Bekircan, O.; Karaoglu, S.A. Synthesis and antimicrobial activity of some 3-alkyl-4-(arylmethyleneamino)-4,5-dihydro-1H-1,2,4-triazol-5-ones. Indian J. Chem. 2005, 44B, 2614–2617. [Google Scholar] [CrossRef]
- Bekircan, O.; Kahveci, B.; Kucuk, M. Synthesis and anticancer evaluation of some new unsymmetrical 3,5-diaryl-4H-1,2,4-triazole derivatives. Tur. J. Chem. 2006, 30, 29–40. [Google Scholar]
- Singh, W.M.; Dash, B.C. Synthesis of some new schiff bases containing thiazole and oxazole nuclei and their fungicidal activity. Pesticides 1988, 22, 33–37. [Google Scholar]
- Payne, P.; Tyman, J.H.P.; Mehet, S.K.; Ninagawa, A. The synthesis of 2-hydroxymethyl derivatives of phenols. J. Chem. Res. 2006, 37, 402–405. [Google Scholar] [CrossRef]

© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Naganagowda, G.; Potgieter, K.; Meijboom, R.; Petsom, A. 5-Pentadecyl-2-((p-tolylimino)methyl)phenol. Molbank 2013, 2013, M804. https://doi.org/10.3390/M804
Naganagowda G, Potgieter K, Meijboom R, Petsom A. 5-Pentadecyl-2-((p-tolylimino)methyl)phenol. Molbank. 2013; 2013(3):M804. https://doi.org/10.3390/M804
Chicago/Turabian StyleNaganagowda, Gadada, Kariska Potgieter, Reinout Meijboom, and Amorn Petsom. 2013. "5-Pentadecyl-2-((p-tolylimino)methyl)phenol" Molbank 2013, no. 3: M804. https://doi.org/10.3390/M804
APA StyleNaganagowda, G., Potgieter, K., Meijboom, R., & Petsom, A. (2013). 5-Pentadecyl-2-((p-tolylimino)methyl)phenol. Molbank, 2013(3), M804. https://doi.org/10.3390/M804