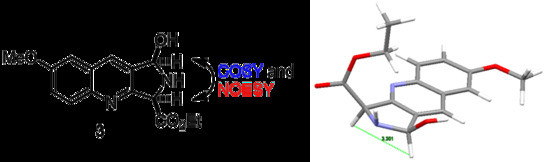
Syn-Ethyl 1-hydroxy-7-methoxy-2,3-dihydro-1H-pyrrolo[3,4-b]quinolone-3-carboxylate HCl Salt
Abstract
:Introduction
Experimental Section
Spectroscopic Data
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Acknowledgments
Author Contributions
Conflicts of Interest
References
- Scannel, J.W.; Blanckley, A.; Boldon, H.; Warrington, B. Diagnosing the decline in pharmaceutical R&D efficiency. Nat. Rev. Drug Disc. 2012, 11, 191–200. [Google Scholar]
- Li, J.J. (Ed.) Heterocyclic Chemistry in Drug Discovery; Wiley: Hoboken, NJ, USA, 2013; ISBN 978-1-118-14890-7.
- Silverman, R.B. The Organic Chemistry of Drug Design and Drug Action; Elsevier Academic Press: Burlington, MA, USA, 2004; ISBN 978-0126437324. [Google Scholar]
- Wermuth, C.G. (Ed.) The Practice of Medicinal Chemistry, 3rd ed.; Elsevier Academic Press: Waltham, MA, USA, 2008; ISBN 978-0-12-374194-3.
- Baumann, M.; Baxendale, I.R.; Ley, S.V.; Nikbin, N. An overview of the key routes to the best selling 5-membered ring heterocyclic pharmaceuticals. Beilstein J. Org. Chem. 2011, 7, 442–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baumann, M.; Baxendale, I.R. An overview of the synthetic routes to the best selling drugs containing 6-membered heterocycles. Beilstein J. Org. Chem. 2013, 9, 2265–2319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vitaku, E.; Smith, D.T.; Njardarson, J.T. Analysis of the structural diversity, substitution patterns, and frequency of nitrogen heterocycles among U.S. FDA approved pharmaceuticals. J. Med. Chem. 2014, 57, 10257–10274. [Google Scholar] [CrossRef] [PubMed]
- Baumann, M.; Baxendale, I.R.; Ley, S.V.; Smith, C.D.; Tranmer, G.K. Fully automated continuous flow synthesis of 4,5-disubstituted oxazoles. Org. Lett. 2006, 8, 5231–5234. [Google Scholar] [CrossRef] [PubMed]
- Baumann, M.; Baxendale, I.R.; Ley, S.V. Synthesis of 3-Nitropyrrolidines via dipolar cycloaddition reactions using a modular flow reactor. Synlett 2010, 5, 749–752. [Google Scholar] [CrossRef]
- Baumann, M.; Baxendale, I.R.; Kirschning, A.; Ley, S.V.; Wegner, J. Synthesis of highly substituted nitropyrrolidines, nitropyrrolizines and nitropyrroles via multicomponent-multistep sequences within a flow reactor. Heterocycles 2010, 82, 1297–1316. [Google Scholar] [CrossRef]
- Baumann, M.; Baxendale, I.R.; Kuratli, C.; Ley, S.V.; Martin, R.E.; Schneider, J. Synthesis of a drug-like focused library of trisubstituted pyrrolidines using integrated flow chemistry and batch methods. ACS Comb. Sci. 2011, 13, 405–415. [Google Scholar] [CrossRef] [PubMed]


© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baumann, M.; Baxendale, I.R. Syn-Ethyl 1-hydroxy-7-methoxy-2,3-dihydro-1H-pyrrolo[3,4-b]quinolone-3-carboxylate HCl Salt. Molbank 2015, 2015, M846. https://doi.org/10.3390/M846
Baumann M, Baxendale IR. Syn-Ethyl 1-hydroxy-7-methoxy-2,3-dihydro-1H-pyrrolo[3,4-b]quinolone-3-carboxylate HCl Salt. Molbank. 2015; 2015(1):M846. https://doi.org/10.3390/M846
Chicago/Turabian StyleBaumann, Marcus, and Ian R. Baxendale. 2015. "Syn-Ethyl 1-hydroxy-7-methoxy-2,3-dihydro-1H-pyrrolo[3,4-b]quinolone-3-carboxylate HCl Salt" Molbank 2015, no. 1: M846. https://doi.org/10.3390/M846
APA StyleBaumann, M., & Baxendale, I. R. (2015). Syn-Ethyl 1-hydroxy-7-methoxy-2,3-dihydro-1H-pyrrolo[3,4-b]quinolone-3-carboxylate HCl Salt. Molbank, 2015(1), M846. https://doi.org/10.3390/M846