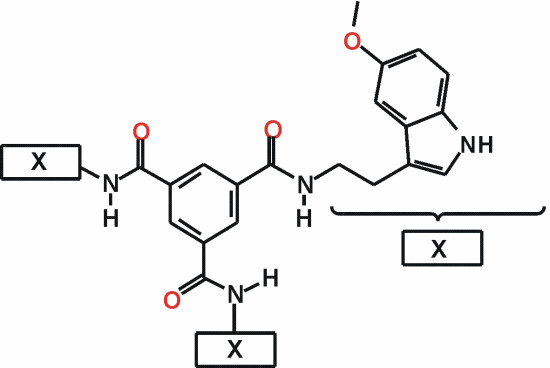
N,N',N"-Tris[(5-methoxy-1H-indol-3-yl)ethyl]benzene-1,3,5-tricarboxamide
Abstract
:Introduction
Experimental Section
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4References and Notes
- For examples, see: Kubik, S. Anion recognition in water. Chem. Soc. Rev. 2010, 39, 3648–3663. [Google Scholar] [CrossRef] [PubMed]
- Gale, P.A. Anion receptor chemistry: highlights from 2008 and 2009. Chem. Soc. Rev. 2010, 39, 3746–3771. [Google Scholar] [CrossRef] [PubMed]
- Kataev, E.A.; Müller, C. Recent advances in molecular recognition in water: artificial receptors and supramolecular catalysis. Tetrahedron 2014, 70, 137–167. [Google Scholar]
- Mazik, M. Molecular recognition of carbohydrates by acyclic receptors employing noncovalent interactions. Chem. Soc. Rev. 2009, 38, 935–956. [Google Scholar] [CrossRef] [PubMed]
- Mazik, M. Recent developments in the molecular recognition of carbohydrates by artificial receptors. RSC Adv. 2012, 2, 2630–2642. [Google Scholar] [CrossRef]
- Mazik, M.; Kuschel, M. Highly effective acyclic carbohydrate receptors consisting of aminopyridine, imidazole, and indole recognition units. Chem. Eur. J. 2008, 14, 2405–2419. [Google Scholar] [CrossRef] [PubMed]
- Mazik, M.; Hartmann, A. Recognition properties of receptors consisting of imidazole and indole recognition units towards carbohydrates. Beilstein J. Org. Chem. 2010, 6. No 9. [Google Scholar] [CrossRef]
- Sonnenberg, C.; Hartmann, A.; Mazik, M. Molecular recognition of carbohydrates: Evaluation of the binding properties of pyrazole-based receptors and their comparison with imidazole- and indole-based systems. Nat. Prod. Commun. 2012, 7, 321–326. [Google Scholar] [PubMed]
- Rosien, J.-R.; Seichter, W.; Mazik, M. Trimethoxybenzene- and trimethylbenzene-based compounds bearing imidazole, indole and pyrrole groups as recognition units: Synthesis and evaluation of the binding properties towards carbohydrates. Org. Biomol. Chem. 2013, 11, 6569–6579. [Google Scholar] [CrossRef] [PubMed]
- Koch, N.; Rosien, J.-R.; Mazik, M. Synthesis of Compounds Based on a Dimesitylmethane Scaffold and Representative Binding Studies Showing Di- vs. Monosaccharide Preference. Tetrahedron 2014, 70, 8758–8767. [Google Scholar] [CrossRef]
- For examples, see: Lis, H.; Sharon, N. Lectins; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2003. [Google Scholar]
- Ito, K.; Umetsu, T.; Kamimura, A. Indole- and amide-based tripodal anion receptor: High affinity towards a hydrogen sulfate. Trends Heterocycl. Chem. 2008, 13, 69–73. [Google Scholar]
- For a review on recognition of ammonium ions, see: Späth, A.; König, B. Molecular recognition of organic ammonium ions in solution using synthetic receptors. Beilstein J. Org. Chem. 2010, 6. No 32. [Google Scholar]
- Hynes, M.J. EQNMR: A computer program for the calculation of stability constants from nuclear magnetic resonance chemical shift data. J. Chem. Soc. Dalton Trans. 1993, 311–312. [Google Scholar] [CrossRef]


© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmidt, U.; Stapf, M.; Mazik, M. N,N',N"-Tris[(5-methoxy-1H-indol-3-yl)ethyl]benzene-1,3,5-tricarboxamide. Molbank 2015, 2015, M850. https://doi.org/10.3390/M850
Schmidt U, Stapf M, Mazik M. N,N',N"-Tris[(5-methoxy-1H-indol-3-yl)ethyl]benzene-1,3,5-tricarboxamide. Molbank. 2015; 2015(2):M850. https://doi.org/10.3390/M850
Chicago/Turabian StyleSchmidt, Ute, Manuel Stapf, and Monika Mazik. 2015. "N,N',N"-Tris[(5-methoxy-1H-indol-3-yl)ethyl]benzene-1,3,5-tricarboxamide" Molbank 2015, no. 2: M850. https://doi.org/10.3390/M850
APA StyleSchmidt, U., Stapf, M., & Mazik, M. (2015). N,N',N"-Tris[(5-methoxy-1H-indol-3-yl)ethyl]benzene-1,3,5-tricarboxamide. Molbank, 2015(2), M850. https://doi.org/10.3390/M850