4-Chloro-6-ethoxy-2-(methylthio)pyrimidine
Abstract
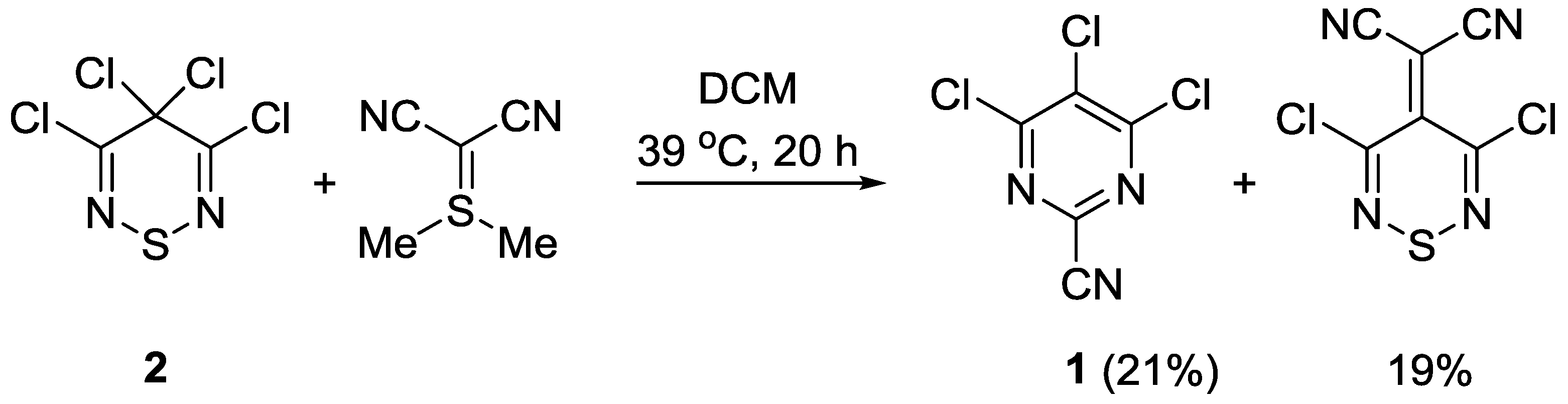
:1. Introduction
2. Results and Discussion
3. Materials and Methods
4-Chloro-6-ethoxy-2-(methylthio)pyrimidine (5)
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rewcastle, G.W. Pyrimidines and their Benzo Derivatives. In Comprehensive Heterocyclic Chemistry III; Katritzky, A.R., Ramsden, C.A., Scriven, E.F.V., Taylor, R.J.K., Eds.; Pergamon Press: Oxford, UK, 2008; Volume 8, pp. 120–252. [Google Scholar]
- Amr, A.E.; Nermien, M.S.; Abdulla, M.M. Synthesis, reactions, and anti-inflammatory activity of heterocyclic systems fused to a thiophene moiety using citrazinic acid as synthon. Monatsh. Chem. 2007, 138, 699–707. [Google Scholar] [CrossRef]
- Desai, K.; Patel, R.; Chikhalia, K. Synthesis of pyrimidine based thiazolidinones and azetidinones: Antimicrobial and antitubercular agents. Ind. J. Chem. 2006, 45, 773–778. [Google Scholar]
- Fujiwara, N.; Nakajima, T.; Ueda, Y.; Fujita, H.; Kawakami, H. Novel piperidinylpyrimidine derivatives as inhibitors of HIV-1 LTR activation. Bioorg. Med. Chem. 2008, 16, 9804–9816. [Google Scholar] [CrossRef] [PubMed]
- Gorlitzer, K.; Herbig, S.; Walter, R.D. Indeno[1,2-d]pyrimidin-4-yl-amines. Pharmazie 1997, 52, 670–672. [Google Scholar]
- Wagner, E.; Al-Kadasi, K.; Zimecki, M.; Sawka-Dobrowolska, W. Synthesis and pharmacological screening of derivatives of isoxazolo[4,5-d]pyrimidine. Eur. J. Med. Chem. 2008, 43, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Kalogirou, A.S.; Manoli, M.; Koutentis, P.A. Synthesis of N-aryl-3,5-dichloro-4H-1,2,6-thiadiazin-4-imines from 3,4,4,5-tetrachloro-4H-1,2,6-thiadiazine. Org. Lett. 2015, 17, 4118–4121. [Google Scholar] [CrossRef] [PubMed]
- Kalogirou, A.S.; Koutentis, P.A. A qualitative comparison of the reactivities of 3,4,4,5-tetrachloro-4H-1,2,6-thiadiazine and 4,5-dichloro-1,2,3-dithiazolium chloride. Molecules 2015, 20, 14576–14594. [Google Scholar] [CrossRef] [PubMed]
- Kalogirou, A.S.; Koutentis, P.A. Regioselective geminal dichloride reactivity of 3,4,4,5-tetrachloro-4H-1,2,6-thiadiazine: access to 4,4-dioxo- and dithio-ketals. Tetrahedron Lett. 2016, 57, 203–205. [Google Scholar] [CrossRef]
- Kalogirou, A.S.; Koutentis, P.A. Substitution chemistry of 3,5-dichloro-4H-1,2,6-thiadiazine 4,4-ketals. Tetrahedron Lett. 2016, 57, 3307–3310. [Google Scholar] [CrossRef]
- Koutentis, P.A.; Rees, C.W. Reaction of tetracyanoethylene with SCl2; new molecular rearrangements. J. Chem. Soc. Perkin Trans. 1 2000, 1089–1094. [Google Scholar] [CrossRef]
- Hyun, S.Y.; Jung, S.O.; Lee, R.N. An Electroluminescent Compound and an Electroluminescent Device Comprising the Same. KR2015/124637 A, 25 November 2015. [Google Scholar]
- Liu, J.; Fitzgerald, A.; Mani, N. Facile assembly of fused benzo[4,5]furo Heterocycles. J. Org. Chem. 2008, 73, 2951–2954. [Google Scholar] [CrossRef] [PubMed]
- Mabuko, Y. Triallylisocyanurate, Triallylcyanurate, and Process for Production of Triallylisocyanurate. EP2436677 A1, 4 April 2012. [Google Scholar]
- Chen, Y.; Kanouni, T.; Kaldor, S.; Stafford, J.A.; Veal, J.M. Inhibitors of Lysine Specific Demethylase-1. WO2015/168466 A1, 5 November 2015. [Google Scholar]
- Ladd, D.L. Synthesis of some substituted guanidinopyrimidines and their structural assignment by 13C and 1H-NMR. J. Het. Chem. 1982, 19, 917–921. [Google Scholar] [CrossRef]
- Kukla, M.J.; Ludovici, D.W.; Kavash, R.W.; de Corte, B.L.D.; Heeres, J.; Janssen, P.A.J.; Koymans, L.M.H.; de Jonge, M.R.; van Aken, K.J.A.; Krief, A. Prodrugs of HIV Replication Inhibiting Pyrimidines. EP1282607 B1, 12 February 2003. [Google Scholar]
- Raboisson, P.; Belfrage, A.; Classon, B.; Lindquist, K.; Nilsson, K.; Rosenquist, A.; Samuelson, B.; Wahling, H. Pyrimidine Substituted Macrocyclic HCV Inhibitors. WO2008/095999A1, 14 August 2008. [Google Scholar]
- Bayne, C.D.; Johnson, A.T.; Lu, S.-P.; Mohan, R.; Nyman, M.C.; Schweiger, E.J.; Stevens, W.C.; Wang, H.; Xie, Y. Modulators of LXR. US20050080111 A1, 14 April 2005. [Google Scholar]
- Seganish, W.M.; Fischmann, T.O.; Sherborne, B.; Matasi, J.; Lavey, B.; McElroy, W.T.; Tulshian, D.; Tata, J.; Sondey, C.; Garlisi, C.G.; et al. Discovery and Structure Enabled Synthesis of 2,6-Diaminopyrimidin-4-one IRAK4 Inhibitors. ACS Med. Chem. Lett. 2015, 6, 942–947. [Google Scholar] [CrossRef] [PubMed]
- Woods, K.W.; Lai, C.; Miyashiro, J.M.; Tong, Y.; Florjancic, A.S.; Han, E.K.; Soni, N.; Shi, Y.; Lasko, L.; Leverson, J.D.; et al. Aminopyrimidinone Cdc7 Kinase Inhibitors. Bioorg. Med. Chem. Lett. 2012, 22, 1940–1943. [Google Scholar] [CrossRef] [PubMed]
- Tohyama, Y.; Sanemitsu, Y. Uracil Compounds and Use Thereof. US6537948 B1, 25 March 2003. [Google Scholar]
- Kanno, H.; Kubota, Y.; Sato, T.; Arahira, M. 2-Benzyloxy-4-phenoxypyrimidine Derivative, Processes for Producing the Derivative and Herbicidal Composition Containing the Derivative. US5723412 A1, 3 March 1998. [Google Scholar]
- Kobayashi, K.; Ono, R.; Yuba, S.; Hiyoshi, H.; Umezu, K. Two-step synthesis of 5-hydroxy-5,7-dihydro-6H-pyrrolo[2,3-d]pyrimidin-6-one derivatives from 4-chloro-6-methoxy-2-(methylsulfanyl)pyrimidine. Heterocycles 2015, 91, 1177–1185. [Google Scholar] [CrossRef]
- Kobayashi, K.; Kuroda, M.; Tanaka, N.; Yokoi, Y.; Kobayashi, A.; Hiyoshi, H.; Umezu, K. A simple method for the preparation of pyrimido[4,5-d]pyrimidine-2,4(1H,3H)-dithione derivatives. Heterocycles 2014, 89, 1933–1939. [Google Scholar] [CrossRef]

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalogirou, A.S.; Koutentis, P.A. 4-Chloro-6-ethoxy-2-(methylthio)pyrimidine. Molbank 2017, 2017, M923. https://doi.org/10.3390/M923
Kalogirou AS, Koutentis PA. 4-Chloro-6-ethoxy-2-(methylthio)pyrimidine. Molbank. 2017; 2017(1):M923. https://doi.org/10.3390/M923
Chicago/Turabian StyleKalogirou, Andreas S., and Panayiotis A. Koutentis. 2017. "4-Chloro-6-ethoxy-2-(methylthio)pyrimidine" Molbank 2017, no. 1: M923. https://doi.org/10.3390/M923
APA StyleKalogirou, A. S., & Koutentis, P. A. (2017). 4-Chloro-6-ethoxy-2-(methylthio)pyrimidine. Molbank, 2017(1), M923. https://doi.org/10.3390/M923






