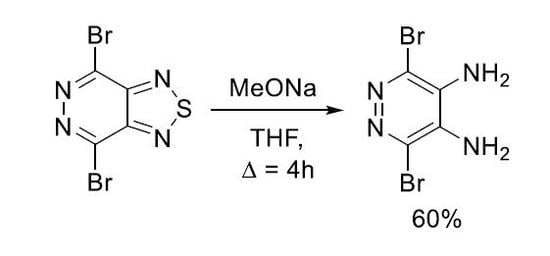
3,6-Dibromopyridazine-4,5-diamine
Abstract
:1. Introduction
2. Results and Discussion
3. Experimental Section
3.1. General Information
3.2. Synthesis of 3,6-Dibromopyridazine-4,5-diamine 2
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Carella, A.; Borbone, F.; Centore, R. Research Progress on Photosensitizers for DSSC. Front. Chem. 2018, 6, 481. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.P.; Li, C.T.; Ho, K.C. Use of organic materials in dye-sensitized solar cells. Mater. Today 2017, 20, 267–283. [Google Scholar] [CrossRef] [Green Version]
- Knyazeva, E.A.; Rakitin, O.A. Influence of structural factors on the photovoltaic properties of dye-sensitized solar cells. Russ. Chem. Rev. 2016, 85, 1146–1183. [Google Scholar] [CrossRef]
- Konstantinova, L.S.; Knyazeva, E.A.; Rakitin, O.A. Recent Developments in the Synthesis and Applications of 1,2,5-Thia- and Selenadiazoles. A Review. Org. Prep. Proc. Int. 2014, 46, 475–544. [Google Scholar] [CrossRef]
- Chulanova, E.A.; Semenov, N.A.; Pushkarevsky, N.A.; Gritsan, N.P.; Zibarev, A.V. Charge-transfer chemistry of chalcogen-nitrogen π-heterocycles. Mendeleev Commun. 2018, 28, 453–460. [Google Scholar] [CrossRef]
- Lonchakov, A.V.; Rakitin, O.A.; Gritsan, N.P.; Zibarev, A.V. Breathing some new life into an old topic: chalcogen-nitrogen π-heterocycles as electron acceptors. Molecules 2013, 18, 9850–9900. [Google Scholar] [CrossRef] [PubMed]
- Gritsan, N.P.; Zibarev, A.V. Chalcogen-nitrogen π-heterocyclic radical anion salts: the synthesis and properties. Russ. Chem. Bull. 2011, 60, 2131–2140. [Google Scholar] [CrossRef]
- Rakitin, O.A.; Zibarev, A.V. Recent Progress in Synthesis and Applications of 5-Membered Chalcogen-Nitrogen π-Heterocycles with Three Heteroatoms. Asian J. Org. Chem. 2018, 7, 2397–2416. [Google Scholar] [CrossRef]
- Knyazeva, E.A.; Rakitin, O.A. 4,7-Dibromo-substituted 2,1,3-benzothia(selena, oxa)diazoles and [1,2,5]thia(selena)diazolo[3,4-c]pyridine as building blocks in solar cells components (microreview). Chem. Heterocycl. Comp. 2017, 53, 855–857. [Google Scholar] [CrossRef]
- Chmovzh, T.N.; Knyazeva, E.A.; Mikhalchenko, L.V.; Golovanov, I.S.; Amelichev, S.A.; Rakitin, O.A. Synthesis of 4,7-dibromo derivative of ultrahigh electron-deficient [1,2,5]thiadiazolo[3,4-d]pyridazine heterocycle and its cross-coupling reactions. Eur. J. Org. Chem. 2018. [Google Scholar] [CrossRef]
- Yanai, M.; Kinoshita, T.; Takeda, S.; Sadaki, H.; Watanabe, H. Synthesis of pyridazine derivatives. XII. Synthesis of 4,5-diaminopyridazine derivatives. Chem. Pharm. Bull. 1970, 18, 1680–1685. [Google Scholar] [CrossRef]
- Chmovzh, T.N.; Knyazeva, E.A.; Lyssenko, K.A.; Popov, V.V.; Rakitin, O.A. Safe synthesis of 4,7-dibromo[1,2,5]thiadiazolo[3,4-d]pyridazine and its SNAr reactions. Molecules 2018, 23, 2576. [Google Scholar] [CrossRef] [PubMed]
- Koutentis, P.A. 1,2,5-Thiadiazoles. In Comprehensive Heterocyclic Chemistry III; Katritzky, A.R., Ramsden, C.A., Scriven, E.F.V., Taylor, R.J.K., Eds.; Elsevier: Oxford, UK, 2008; Volume 5, pp. 516–564. [Google Scholar] [CrossRef]

| Entry | Solvent | Reagent | Temperature, °C | Time, h | Yield, % | |
|---|---|---|---|---|---|---|
| 2 | 3 | |||||
| 1 | EtOH | NaBH4CoCl2·6H2O (cat.) | 78 | 3 | 0 | 0 |
| 2 | THF | LAH | 66 | 3 | 0 | 0 |
| 3 | MeOH | MeONa | 64 | 5 | traces | 70 |
| 4 | THF | MeONa | 66 | 4 | 60 | 0 |
| 5 | MeCN | MeONa | 81 | 4 | 55 | 0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chmovzh, T.N.; Knyazeva, E.A.; Ustimenko, O.O.; Rakitin, O.A. 3,6-Dibromopyridazine-4,5-diamine. Molbank 2019, 2019, M1053. https://doi.org/10.3390/M1053
Chmovzh TN, Knyazeva EA, Ustimenko OO, Rakitin OA. 3,6-Dibromopyridazine-4,5-diamine. Molbank. 2019; 2019(1):M1053. https://doi.org/10.3390/M1053
Chicago/Turabian StyleChmovzh, Timofey N., Ekaterina A. Knyazeva, Olga O. Ustimenko, and Oleg A. Rakitin. 2019. "3,6-Dibromopyridazine-4,5-diamine" Molbank 2019, no. 1: M1053. https://doi.org/10.3390/M1053
APA StyleChmovzh, T. N., Knyazeva, E. A., Ustimenko, O. O., & Rakitin, O. A. (2019). 3,6-Dibromopyridazine-4,5-diamine. Molbank, 2019(1), M1053. https://doi.org/10.3390/M1053