Abstract
A new thiophene-substituted terpyridine derivative has been prepared and characterized. This ligand features a thiophene heterocycle (as an electrochemically polymerizable unit) as well as two chlorine atoms for further functionalization.
1. Introduction
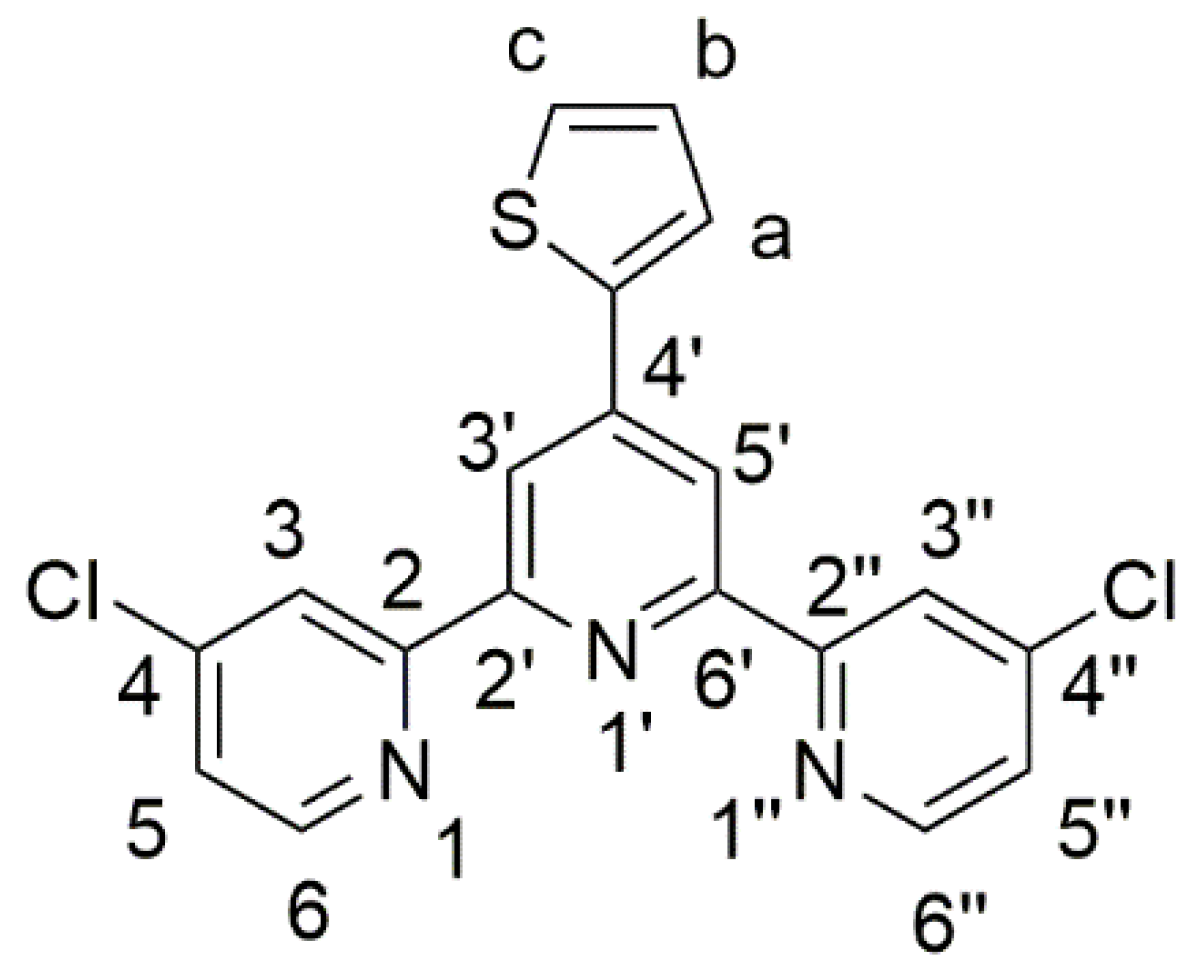
Terpyridine (terpy) derivatives (especially 2,2′:6′,2″-terpyridines) and their complexes have been widely studied [1]. In fact, an important number of terpyridine-based compounds can be prepared by varying the substitution pattern onto the terpyridine scaffold and/or the nature of the complexed metal. Terpyridines and their complexes have found a vast range of applications such as sensitizers in solar cells [2], catalysts [3], materials for water treatment [4], electrochromic materials [5], or as chromophores [6], just to name a few. Terpyridine complexes that are substituted with a thiophene ring are interesting compounds because they also find many possible applications in material science [7] or medicinal chemistry and biology [8,9], for example. Especially, the thiophene heterocycle allows for the formation of polymeric materials through electrochemical polymerization. The so obtained terpyridine-containing materials have shown potential uses as sensors [10] or as electrochromic materials [11], for example. Therefore, the synthesis of new terpy derivatives that bear a pendant thiophene ring is still of interest. This article follows our previous work on furan-containing terpy [12] and describes the synthesis and characterization of 4,4″-dichloro-4′-(2-thienyl)-2,2′:6′,2″-terpyridine (1) (Figure 1). This ligand not only features a thiophene ring but also possesses two chlorine atoms on the outer pyridine rings, which allow for future functionalization of this terpyridine.
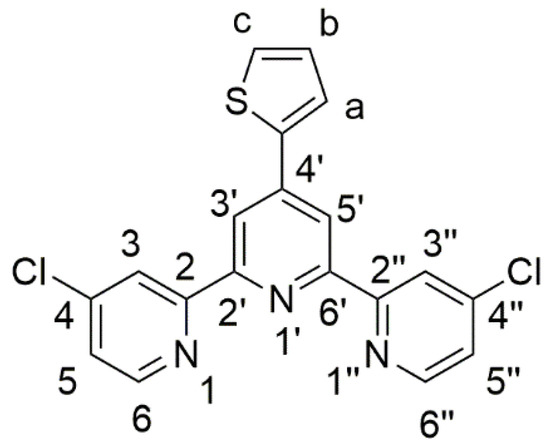
Figure 1.
Structure and atom numbering of 4,4″-dichloro-4′-(2-thienyl)-2,2′:6′,2″-terpyridine (1).
2. Results and Discussion
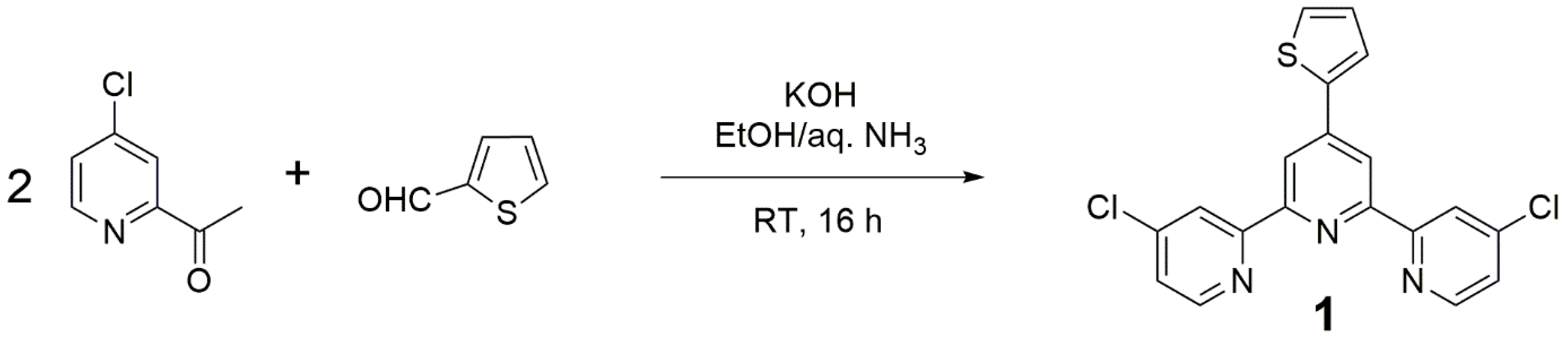
Amongst all methods available for the preparation of terpyridine derivatives [13,14,15], the one from Wang and Hanan [16] was selected, although sometimes this method fails in producing terpyridine derivatives [17]. Thus 1 was simply prepared by mixing 4-chloro-2-acetylpyridine [18], thiophene-2-carboxaldehyde, and potassium hydroxide in ethanol and aqueous ammonia (Figure 2).
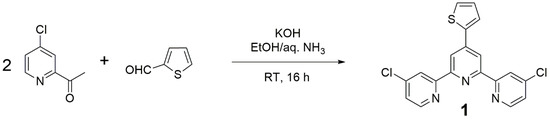
Figure 2.
Preparation of terpyridine (Compound 1).
Crude 1 precipitated from the reaction mixture and was isolated by simple filtration. At this point, the material collected was sufficiently pure (>98% by quantitative NMR [19]) to be used for further experiments (e.g., preparation of metal complexes) without purification.
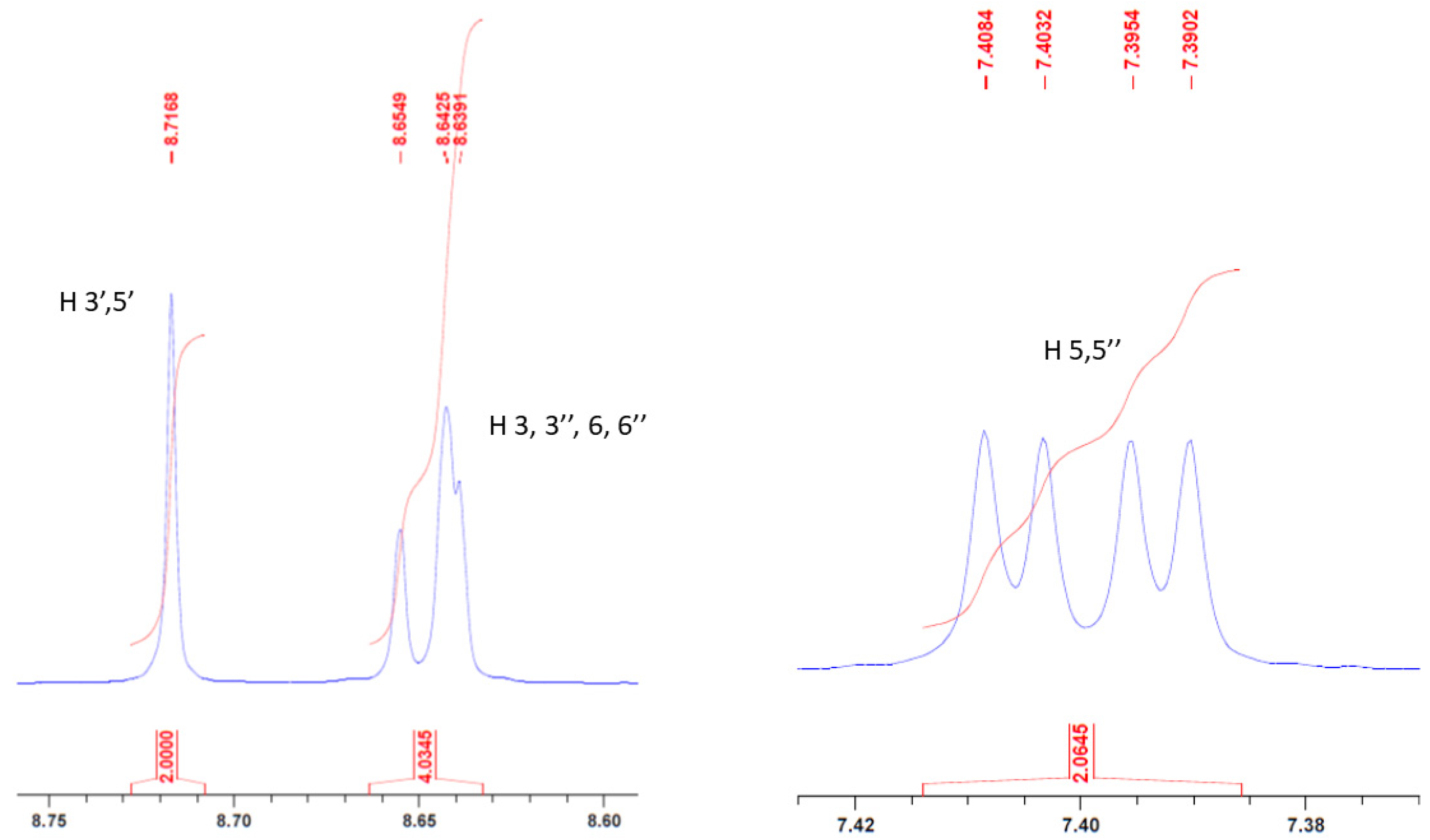
The identity of compound 1 was confirmed by different analytical techniques. Firstly, NMR spectra (1H and 13C) agreed with the chemical structure. Especially, the singlet for proton 3′ and 5′ was clearly seen at δ = 8.72 ppm. Furthermore, signals for protons 3, 3″, 6, and 6″ were merged into a multiplet while protons 5 and 5″ appeared as a doublet of doublet (Figure 3). Such an 1H NMR pattern has been already observed for 4,4″-dichloro-2,2′:6′,2″-terpyridine [20].
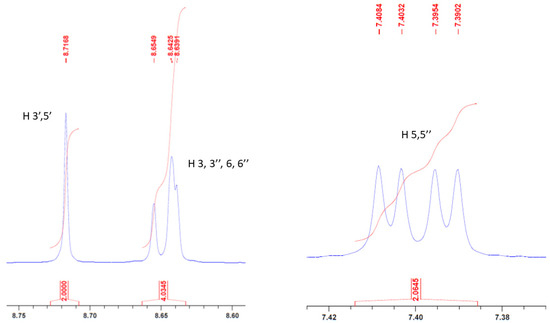
Figure 3.
Part of NMR spectrum showing signals for protons (3′,5′), (3,3″,6′,6″), and (5,5″).
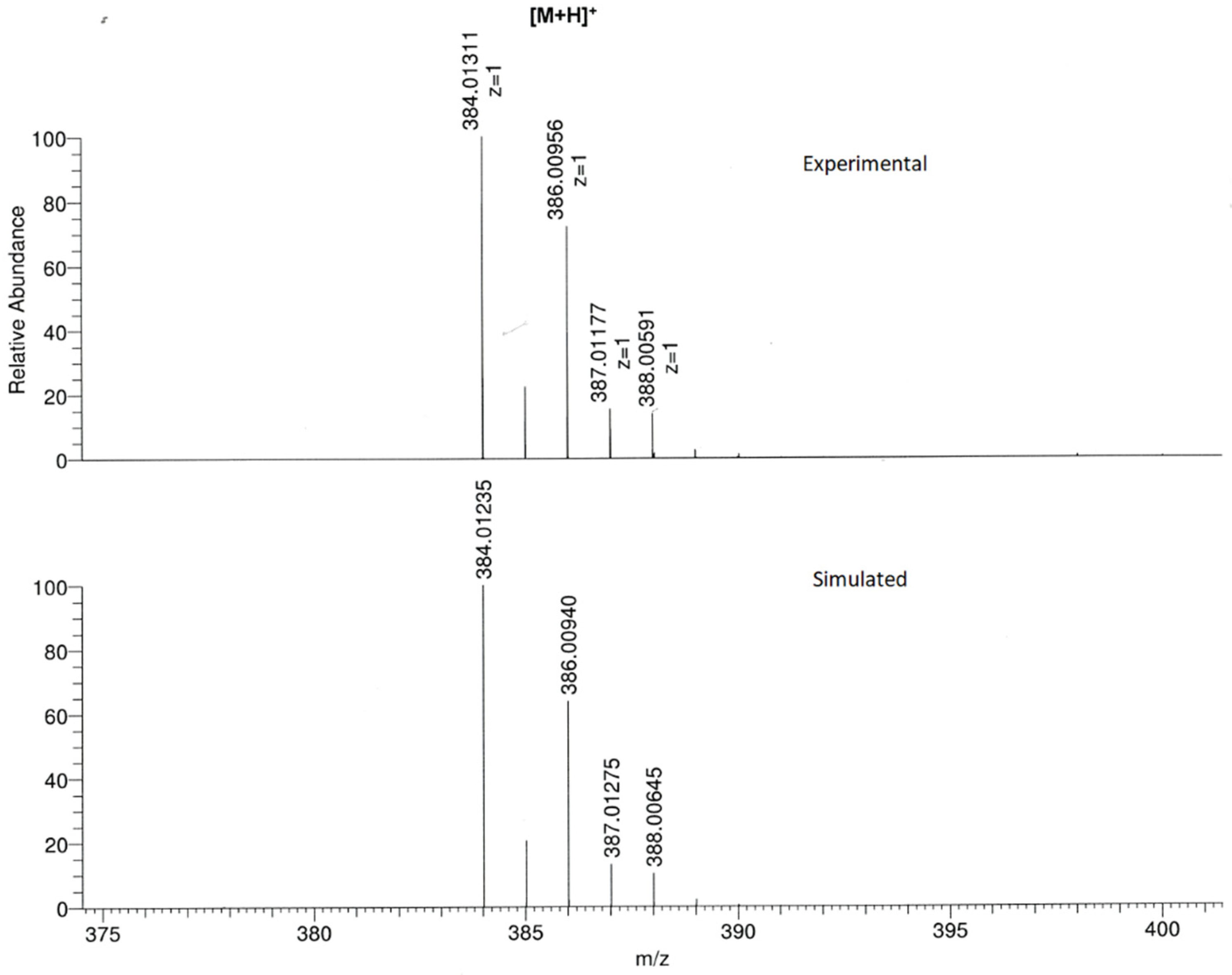
Additionally, the formation of the title compound was confirmed by HR-MS (Figure S3) since the [M + H]+ ion peak, as well as the isotopic distribution, corresponded to molecular formula (C19H12Cl2N3S+). In fact, the experimental recorded spectrum was in good agreement with the simulated one (Figure 4).
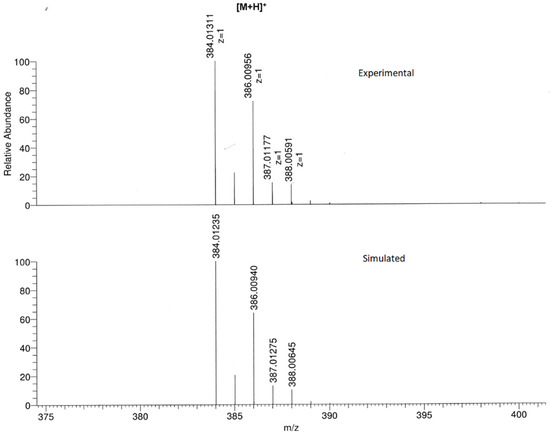
Figure 4.
Experimental (top) and simulated (bottom) mass spectra for [M + H]+ ion.
3. Materials and Methods
The 4-chloropicolinonitrile was purchased from ABCR Gmbh (Karlsruhe, Germany) and used as received. Methylmagnesium chloride (20% solution in THF) was purchased from Merck Schuchardt (Hohenbrunn, Germany). This Grignard solution was titrated prior to use with salicylaldehyde phenylhydrazone according to literature [21] and the measured concentration was 2.9 M. All other chemicals were purchased from ACROS organics (Geel, Belgium). Anhydrous THF was obtained from a solvent purification system (Innovative Technology, Amesbury, MA, USA). The 4-chloro-2-acetylpyridine was prepared according to the literature [18]. 1H and 13C NMR spectra were recorded on a Brucker AC 400 (Bruker, Wissembourg, France) at 400 and 100 MHz, respectively, using CDCl3 as a solvent. Melting point was recorded with a Stuart SMP 10 melting point apparatus (Bibby Sterilin, Stone, UK) and was uncorrected. Elemental analysis was performed at Service d’Analyses Elementaires, UMR 7565 CNRS, Vandoeuvre-les-Nancy, France. HR-MS was recorded at Sayence SATT, Dijon, France.
3.1. Preparation of 4-Chloro-2-acetylpyridine
To a solution of methylmagnesium chloride (50 mL; 0.145 mol, excess) at 0 °C under argon was added dropwise a solution of 4-chloropicolinonitrile (5.00 g; 0.036 mol) in anhydrous THF (120 mL). The reaction mixture was stirred at room temperature for 6 h. The solution was then poured cautiously onto a saturated ammonium chloride aqueous solution (120 mL) at 0 °C, and the pH of the solution was adjusted to 1 with concentrated hydrochloric acid. The resulting solution was stirred at room temperature overnight and then neutralized with 25% aqueous ammonia. The aqueous solution was extracted with ethyl acetate (6 × 50 mL). Organic layers were combined, washed with brine (2 × 100 mL), dried over sodium sulphate, and concentrated. The crude product was purified by flash chromatography with cyclohexane/ethylacetate as eluent (100:0 to 95:5). Pure product was obtained as a pale-yellow oil (2.58 g; 50%). Spectroscopic data agreed with those reported in the literature [18].
3.2. Preparation of 4,4″-Dichloro-4′-(2-thienyl)-2,2′:6′,2″-terpyridine
To a solution of 4-chloro-2-acetylpyridine (3.11 g; 20 mmol) in ethanol (50 mL) was added thiophene-2-carboxaldehyde (1.12 g; 10 mmol), 85% potassium hydroxide pellets (1.54 g; 24 mmol), and 25% aqueous ammonia (30 mL). The reaction mixture was stirred at room temperature for 16 h. The solid was then filtered on a glass sintered funnel and washed with ice-cold 50% ethanol until washings were colorless. The product was dried under vacuum over phosphorus pentoxide. Compound 1 was obtained as a beige solid (2.20 g; 57%). Mp = 216–219 °C (dec.). 1H NMR (CDCl3, 400 MHz), δ (ppm): 8.72 (s, 2H, H3′, 5′), 8.64–8.65 (m, 4H, H3, 3″, 6, 6″), 7.78 (dd, 1H, Ha, J = 4.0 Hz, J = 1.0 Hz), 7.49 (dd, 1H, Hc, J = 5.0 Hz, J = 1.0 Hz), 7.40 (dd, 2H, H5, 5″, J = 5.2 Hz, J = 2.1 Hz), 7.20 (dd, 1H, Hb, J = 5.0 Hz, J = 4.0 Hz). 13C NMR (CDCl3, 100 MHz), δ (ppm): 157.3, 155.0, 150.0, 145.2, 143.7, 141.4, 128.4, 127.5, 126.1, 124.2, 121.6, 118.0. Elemental analysis for C19H11Cl2N3S: C, 59.38; H, 2.89; N, 10.93; S, 8.34. Found C, 58.89; H, 2.94; N, 10.59; S, 8.30. HR-MS: calc. for (C19H11Cl2N3S + H)+ 384.01235, found 384.01311.
4. Conclusions
A new thiophene-substituted terpyridine was prepared and characterized. Future work will focus on further functionalizing this ligand owing to the presence of two chlorine atoms onto the outer rings. For instance, these chlorines could be substituted by different nucleophiles as already reported for 4′-chloro-2,2′:6′,2″-terpyridine [22,23,24] or 4,4″-dichloro-2,2′:6′,2″-terpyridine [20,25,26]. Experiments are currently in progress in our laboratory to prepare new terpyridine derivatives by this method.
Supplementary Materials
The following are available online: Figure S1: 1H NMR of 1, Figure S2: 13C NMR of 1, Figure S3: HR-MS of 1, Figure S4: detailed molecular ion peak.
Author Contributions
J.H. conceived and carried out the experiments, analyzed data, and prepared the manuscript. L.G. analyzed data and contributed to manuscript preparation.
Funding
This research was funded by Université de Franche-Comté (CHRYSALIDE Project).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Schubert, U.S.; Hofmeier, H.; Newkome, G.R. Modern Terpyridine Chemistry; Wiley-VCH: Weinheim, Germany, 2006. [Google Scholar]
- Saccone, D.; Magistris, C.; Barbero, N.; Quagliotto, P.; Barolo, C.; Viscardi, G. Terpyridine and Quaterpyridine Complexes as sensitizers for Photovoltaic Applications. Materials 2016, 9, 137. [Google Scholar] [CrossRef] [PubMed]
- Winter, A.; Newkome, G.R.; Schubert, U.S. Catalytic Applications of Terpyridines and their Transition Metal Complexes. ChemCatChem 2011, 3, 1384–1406. [Google Scholar] [CrossRef]
- Oyetade, O.A.; Nyamori, V.O.; Jonnalagadda, S.B.; Martincigh, B.S. Removal of Cd2+ and Hg2+ from aqueous solutions by adsorption onto nitrogen-functionalized carbon nanotubes. Desalin. Water Treat. 2018, 108, 253–267. [Google Scholar] [CrossRef]
- Allan, J.T.S.; Quaranta, S.; Ebralidze, I.I.; Egan, J.G.; Poisson, J.; Laschuk, N.O.; Gaspari, F.; Easton, E.B.; Zenkina, O.V. Terpyridine-Based Monolayer Electrochromic Materials. ACS Appl. Mater. Interfaces 2017, 9, 40438–40445. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, S.S.M.; Besley, M.; Ciarrocchi, C.; Licchelli, M.; Raposo, M.M.M. Terpyridine derivatives functionalized with (hetero)aromatic groups and the corresponding Ru complexes: Synthesis and characterization as SHG chromophores. Dyes Pigment. 2018, 150, 49–58. [Google Scholar] [CrossRef]
- Husson, J.; Knorr, M. 2,2′:6′,2″-Terpyridines Functionalized with Thienyl Substituents: Synthesis and Applications. J. Heterocyclic Chem. 2012, 49, 453–478. [Google Scholar] [CrossRef]
- Qin, Q.-P.; Wang, Z.-F.; Wang, S.-L.; Luo, D.-M.; Zou, B.-Q.; Yao, P.-F.; Tan, M.-X.; Liang, H. In vitro and in vivo antitumor activities of three novel binuclear platinum(II) complexes with 4′-substituted-2,2′:6′,2″-terpyridine ligands. Eur. J. Med. Chem. 2019, 170, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Shao, T.; Fang, B.; Du, W.; Zhang, M.; Liu, J.; Liu, T.; Tian, X.; Zhang, Q.; Wang, A.; et al. Visualization of mitochondrial DNA in living cells with super-resolution microscopy using thiophene-based terpyridine Zn(II) complexes. Chem. Commun. 2018, 54, 11288–11291. [Google Scholar] [CrossRef] [PubMed]
- Savan, E.K.; Koytepe, S.; Pasahan, A.; Erdogdu, G.; Seckin, T. Amperometric Simultaneous Measurement of Copper and Cobalt Ions with Polythiophene Incorporating Pendant Terpyridine Groups. Polym.-Plast. Technol. Eng. 2014, 53, 1817–1824. [Google Scholar] [CrossRef]
- Liang, Y.W.; Strohecker, D.; Lynch, V.; Holliday, B.J.; Jones, R.A. A Thiophene-Containing Conductive Metallopolymer Using an Fe(II) Bis(terpyridine) Core for Electrochromic Materials. ACS Appl. Mater. Interfaces 2016, 8, 34568–34580. [Google Scholar] [CrossRef]
- Husson, J.; Guyard, L. 4′-(5-Methylfuran-2-yl)-2,2′:6′,2″-terpyridine: A new ligand obtained from a biomass-derived aldehyde with potential application in metal-catalyzed reactions. Molbank 2018, 2018, M1032. [Google Scholar] [CrossRef]
- Heller, M.; Schubert, U.S. Syntheses of functionalized 2,2′:6′,2″-terpyridines. Eur. J. Org. Chem. 2003, 6, 947–961. [Google Scholar] [CrossRef]
- Fallahpour, R.A. Synthesis of 4′-substituted-2,2′:6′,2″-terpyridines. Synthesis 2003, 2, 155–184. [Google Scholar] [CrossRef]
- Thompson, A.M.W.C. The synthesis of 2,2′:6′,2″-terpyridine ligands- versatile building blocks for supramolecular chemistry. Coord. Chem. Rev. 1997, 160, 1–52. [Google Scholar] [CrossRef]
- Wang, J.; Hanan, G.S. A Facile Route to Sterically Hindered and Non-Hindered 4′-Aryl-2,2′:6′,2″-Terpyridines. Synlett 2005, 8, 1251–1254. [Google Scholar] [CrossRef]
- Rocco, D.; Housecroft, C.E.; Constable, E.C. Synthesis of Terpyridines: Simple Reactions-What Could Possibly Go Wrong? Molecules 2019, 24, 1799. [Google Scholar] [CrossRef] [PubMed]
- Busto, E.; Gotor-Fernandez, V.; Gotor, V. Biocatalytic preparation of optically active 4-(N,N-dimethylamino)pyridines for application in chemical asymmetric catalysis. Tetrahedron-Asymmetry 2006, 17, 1007–1016. [Google Scholar] [CrossRef]
- Bharti, S.K.; Roy, R. Quantitative 1H NMR spectroscopy. Trac-Trends Anal. Chem. 2012, 35, 5–26. [Google Scholar] [CrossRef]
- Harzmann, G.D.; Neuburger, M.; Mayor, M. 4,4″-Disubstituted Terpyridines and Their Homoleptic FeII Complexes. Eur. J. Inorg. Chem. 2013, 19, 3334–3347. [Google Scholar] [CrossRef]
- Love, B.E.; Jones, E.G. The Use of Salicylaldehyde Phenylhydrazone as an Indicator for the Titration of Organometallic Reagents. J. Org. Chem. 1999, 64, 3755–3756. [Google Scholar] [CrossRef]
- Lowe, G.; Droz, A.S.; Vilaivan, T.; Weaver, G.W.; Tweedale, L.; Pratt, J.M.; Rock, P.; Yardley, V.; Croft, S.L. Cytotoxicity of (2,2′:6′,2″-Terpyridine)platinum(II) Complexes to Leishmania donovani, Trypanosoma cruzi, and Trypanosoma brucei. J. Med. Chem. 1999, 42, 999–1006. [Google Scholar] [CrossRef] [PubMed]
- Bode, S.; Enke, M.; Bose, R.K.; Schacher, F.H.; Garcia, S.J.; van der Zwaag, S.; Hager, M.D.; Schubert, U.S. Correlation between scratch healing and rheological behaviour for terpyridine complex based metallopolymers. J. Mater. Chem. A 2015, 3, 22145–22153. [Google Scholar] [CrossRef]
- Mutai, T.; Cheon, J.-D.; Arita, S.; Araki, K. Phenyl-substituted 2,2′:6′,2″-terpyridine as a new series of fluorescent compounds-their photophysical properties and fluorescence tuning. J. Chem. Soc. Perkin Trans. 2 2001, 1045–1050. [Google Scholar] [CrossRef]
- Brandl, T.; Hoffmann, V.; Pannwitz, A.; Haussinger, D.; Neuburger, M.; Fuhr, O.; Bernhard, S.; Wenger, O.S.; Mayor, M. Chiral macrocyclic terpyridine complexes. Chem. Sci. 2018, 9, 3837–3843. [Google Scholar] [CrossRef] [PubMed]
- Moore, C.M.; Bark, B.; Szymczak, N.K. Simple Ligand Modifications with Pendent OH Groups Dramatically Impact the Activity and Selectivity of Ruthenium Catalysts for Transfer Hydrogenation: The Importance of Alkali Metals. ACS Catal. 2016, 6, 1981–1990. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).