Pyridine-Imidazlolium Salts: Oxidatively Cleavage of N-C Bond via Nitration
Abstract
:1. Introduction
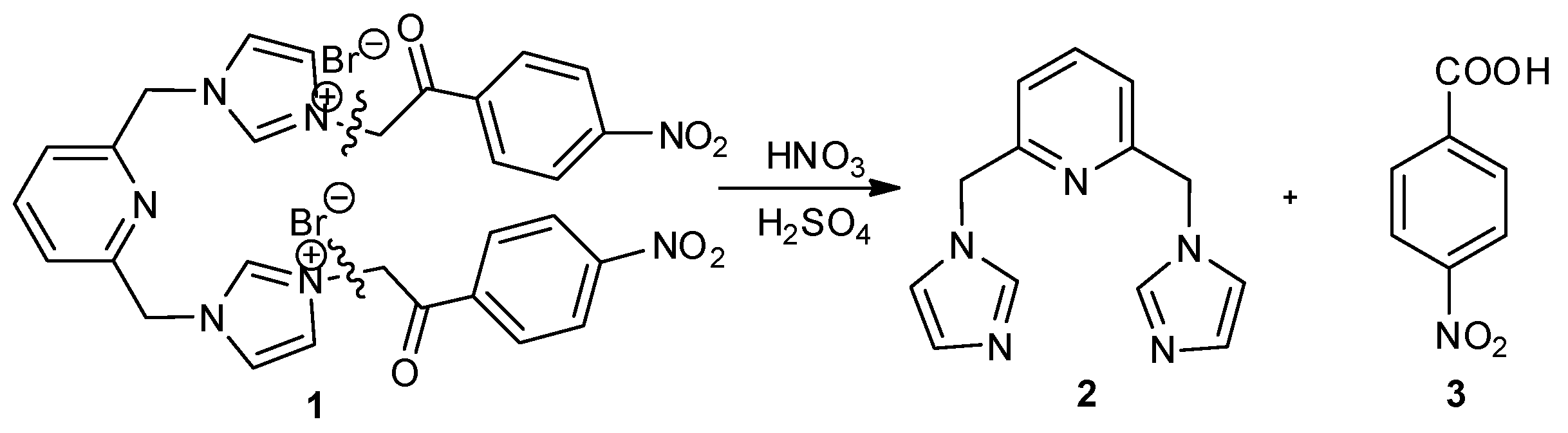
2. Results and Discussion
3. Materials and Methods
3.1. Instrumentation
3.2. Nitration of Pyridine-Imidazolium Salts
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Popovici, L.; Amarandi, R.M.; Mangalagiu, I.I.; Mangalagiu, V.; Danac, R. Synthesis, molecular modelling and anticancer evaluation of new pyrrolo[1,2-b]pyridazine and pyrrolo[2,1-a]phthalazine derivatives. J. Enzym. Inh. Med. Chem. 2019, 34, 230–243. [Google Scholar] [CrossRef] [PubMed]
- Lungu, C.N.; Bratanovici, B.I.; Grigore, M.M.; Antoci, V.; Mangalagiu, I.I. Hybrid imidazole-pyridine derivatives: An approach to novel anticancer DNA intercalators. Curr. Med. Chem. 2018, in press. [Google Scholar] [CrossRef] [PubMed]
- Olaru, A.M.; Vasilache, V.; Danac, R.; Mangalagiu, I.I. Antimycobacterial activity of nitrogen heterocycles derivatives: 7-(pyridine-4-yl)-indolizine derivatives. Part VII. J. Enz. Inhib. Med. Chem. 2017, 32, 1291–1298. [Google Scholar] [CrossRef] [PubMed]
- Mantu, D.; Antoci, V.; Moldoveanu, C.; Zbancioc, G.; Mangalagiu, I.I. Hybrid imidazole (benzimidazole)/pyridine (quinoline) derivatives and evaluation of their anticancer and antimycobacterial activity. J. Enz. Inhib. Med. Chem. 2016, 31, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Mantu, D.; Maftei, D.; Iurea, D.; Ursu, C.; Bejan, V. Synthesis, structure, and in vitro anticancer activity of new polycyclic 1,2-diazines. Med. Chem. Res. 2014, 23, 2909–2915. [Google Scholar] [CrossRef]
- Luca, M.C.; Tura, V.; Mangalagiu, I.I. Considerations concerning design and mechanism of action of a new class of dual DNA intercalators. Med. Hyp. 2010, 75, 627–629. [Google Scholar] [CrossRef] [PubMed]
- Gaba, M.; Mohan, C. Development of drugs based on imidazole and benzimidazole bioactive heterocycles: Recent advances and future directions. Med. Chem. Res. 2016, 25, 173–210. [Google Scholar] [CrossRef]
- Al Matarneh, C.M.; Shova, S.; Mangalagiu, I.I.; Danac, R. Synthesis, structure, antimycobacterial and anticancer evaluation of new pyrrolo-phenanthroline derivatives. J. Enz. Inhib. Med. Chem. 2016, 31, 470–480. [Google Scholar] [CrossRef]
- Danac, R.; Al Matarneh, C.M.; Shova, S.; Daniloaia, T.; Balan, M.; Mangalagiu, I.I. New indolizines with phenanthroline skeleton: Synthesis, structure, antimycobacterial and anticancer evaluation. Bioorg. Med. Chem. 2015, 23, 2318–2327. [Google Scholar] [CrossRef]
- Danac, R.; Managalagiu, I.I. Antimycobacterial activity of nitrogen heterocycles derivatives: Bipyridine derivatives. Part III. Eur. J. Med. Chem. 2014, 74, 664–670. [Google Scholar] [CrossRef]
- Tucaliuc, R.; Cotea, V.; Niculaua, M.; Tuchilus, C.; Mantu, D.; Mangalagiu, I.I. New Pyridazine–Fluorine Derivatives: Synthesis, Chemistry and Biological Activity. Part II. Eur. J. Med. Chem. 2013, 67, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Mantu, D.; Antoci, V.; Mangalagiu, I.I. Design, synthesis and antituberculosis activity of some new pyridazine derivatives: Bis-pyridazine. Part IV. Infect. Disord. Drug. Targets 2013, 13, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Mantu, D.; Luca, M.C.; Moldoveanu, C.; Zbancioc, G.; Mangalagiu, I.I. Synthesis and antituberculosis activity of some new pyridazine derivatives. Part II. Eur. J. Med. Chem. 2010, 45, 5164–5168. [Google Scholar] [CrossRef] [PubMed]
- Butnariu, R.; Mangalagiu, I.I. New pyridazine derivatives: Synthesis, chemistry and biological activity. Bioorg. Med. Chem. 2009, 17, 2823–2829. [Google Scholar] [CrossRef]
- Cucu, D.; Mangalagiu, V.; Amariucai-Mantu, D.; Antoci, V.; Mangalagiu, I.I. Imidazolium ylides: Cycloaddition versus hydrolysis. Studia UBB Chemia 2019, LXIV(3), 59–66. [Google Scholar] [CrossRef]
- Zbancioc, G.; Mangalagiu, I.I.; Moldoveanu, C. Ultrasound assisted synthesis of imidazolium salts: An efficient way to ionic liquids. Ultrason. Sonochem. 2015, 23, 376–383. [Google Scholar] [CrossRef]
- Zbancioc, G.; Zbancioc, A.M.; Mangalagiu, I.I. Ultrasound and microwave assisted synthesis of dihydroxyacetophenone derivatives with or without 1,2-diazine skeleton. Ultrason. Sonochem. 2014, 21, 802–811. [Google Scholar] [CrossRef]
- Zbancioc, G.; Zbancioc, A.M.; Mantu, D.; Miron, A.; Tanase, C.; Mangalagiu, I.I. Ultrasounds assisted synthesis of highly functionalized acetophenone. Rev. Roum. Chim. 2010, 55, 983–987. [Google Scholar]
- Zbancioc, G.; Bejan, V.; Risca, M.; Moldoveanu, C.; Mangalagiu, I.I. Microwave assisted reactions of new azaheterocyles compounds. Molecules 2009, 14, 403–411. [Google Scholar] [CrossRef]
- Mantu, D.; Moldoveanu, C.; Nicolescu, A.; Deleanu, C.; Mangalagiu, I.I. A facile synthesis of pyridazinone derivatives under ultrasonic irradiation. Ultrason. Sonochem. 2009, 16, 452–454. [Google Scholar] [CrossRef]
- Bejan, V.; Moldoveanu, C.; Mangalagiu, I.I. Ultrasounds assisted reactions of steroid analogous of anticipated biological activities. Ultrason. Sonochem. 2009, 16, 312–315. [Google Scholar] [CrossRef] [PubMed]
- Garrison, J.C.; Simons, R.S.; Talley, J.M.; Wesdemiotis, C.; Tessier, C.A.; Youngs, W.J. Synthesis and Structural Characterization of an Imidazolium-Linked Cyclophane and the Silver Complex of an N-Heterocyclic Carbene-Linked Cyclophane. Organometallics 2001, 20, 1276–1278. [Google Scholar] [CrossRef]
- Available online: https://www.sigmaaldrich.com/catalog/product/sial/72910?lang=en®ion=RO&gclid=EAIaIQobChMI2Lbo_oP55QIVE-R3Ch1CQwsMEAAYASAAEgIiTfD_BwE (accessed on 16 November 2019).
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cucu, D.; Mangalagiu, V. Pyridine-Imidazlolium Salts: Oxidatively Cleavage of N-C Bond via Nitration. Molbank 2019, 2019, M1095. https://doi.org/10.3390/M1095
Cucu D, Mangalagiu V. Pyridine-Imidazlolium Salts: Oxidatively Cleavage of N-C Bond via Nitration. Molbank. 2019; 2019(4):M1095. https://doi.org/10.3390/M1095
Chicago/Turabian StyleCucu (Diaconu), Dumitrela, and Violeta Mangalagiu. 2019. "Pyridine-Imidazlolium Salts: Oxidatively Cleavage of N-C Bond via Nitration" Molbank 2019, no. 4: M1095. https://doi.org/10.3390/M1095
APA StyleCucu, D., & Mangalagiu, V. (2019). Pyridine-Imidazlolium Salts: Oxidatively Cleavage of N-C Bond via Nitration. Molbank, 2019(4), M1095. https://doi.org/10.3390/M1095




