3-(4-Bromophenyl)-4-{[3-hydroxy-6-(hydroxymethyl)-4-oxo-4H-pyran-2-yl](m-tolyl)methyl}isoxazol-5(2H)-one
Abstract
:1. Introduction
2. Results and Discussion
2.1. Research of Electrochemically Induced Multicomponent Synthesis of 3-(4-Bromophenyl)-4-{[3-hydroxy-6-(hydroxymethyl)-4-oxo-4H-pyran-2-yl](m-tolyl)methyl}isoxazol-5(2H)-one 4
2.2. X-ray Diffraction Data
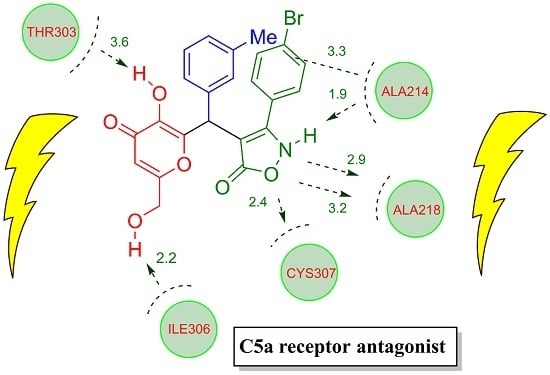
2.3. Docking Studies
3. Materials and Methods
3.1. General Methods
3.2. Electrochemically Induced Multicomponent Synthesis of 3-(4-Bromophenyl)-4-{[3-hydroxy-6-(hydroxymethyl)-4-oxo-4H-pyran-2-yl](m-tolyl)methyl}isoxazol-5(2H)-one 4
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schneider, P.; Schneider, G. A Computational Method for Unveiling the Target Promiscuity of Pharmacologically Active Compounds. Angew. Chem. Int. Ed. 2017, 56, 7971–7994. [Google Scholar] [CrossRef] [Green Version]
- Patchett, A.A.; Nargund, R.P. Chapter 26. Privileged structures. Ann. Rep. Med. Chem. 2000, 35, 289–298. [Google Scholar] [CrossRef]
- Poupaert, J.; Carato, P.; Colacino, E.; Yous, S. 2(3H)-Benzoxazolone and bioisosters as “privileged scaffold” in the design of pharmacological probes. Curr. Med. Chem. 2005, 12, 877–885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reddy, B.V.S.; Reddy, M.R.; Madan, C.; Kumar, K.P.; Rao, M.S. Indium(III) chloride catalyzed three-component coupling reaction: A novel synthesis of 2-substituted aryl(indolyl)kojic acid derivatives as potent antifungal and antibacterial agents. Bioorg. Med. Chem. Lett. 2010, 20, 7507–7511. [Google Scholar] [CrossRef]
- Sharma, D.K.; Pandey, J.; Tamrakar, A.K.; Mukherjee, D. Synthesis of heteroaryl/aryl kojic acid conjugates as stimulators of glucose uptake by GLUT4 translocation. Eur. J. Med. Chem. 2014, 85, 727–736. [Google Scholar] [CrossRef]
- Nayak, D.; Katoch, A.; Sharma, D.; Faheem, M.M.; Chakraborty, S.; Sahu, P.K.; Chikan, N.A.; Amin, H.; Gupta, A.P.; Gandhi, S.G.; et al. Indolylkojyl methane analogue IKM5 potentially inhibits invasion of breast cancer cells via attenuation of GRP78. Breast Cancer Res. Treat. 2019, 177, 307–323. [Google Scholar] [CrossRef]
- Lucas, S.D.; Gonçalves, L.M.; Carvalho, L.A.R.; Correia, H.F.; Da Costa, E.M.R.; Guedes, R.A.; Moreira, R.; Guedes, R.C. Optimization of O3-Acyl Kojic Acid Derivatives as Potent and Selective Human Neutrophil Elastase Inhibitors. J. Med. Chem. 2013, 56, 9802–9806. [Google Scholar] [CrossRef]
- Ishioka, T.; Tanatani, A.; Nagasawa, K.; Hashimoto, Y. Anti-androgens with full antagonistic activity toward human prostate tumor LNCaP cells with mutated androgen receptor. Bioorg. Med. Chem. Lett. 2003, 13, 2655–2658. [Google Scholar] [CrossRef]
- Patrizia, D.; Carbone, A.; Barraja, P.; Kelter, G.; Fiebig, H.H.; Cirrincione, G. Synthesis and antitumor activity of 2,5-bis(3’-indolyl)-furans and 3,5-bis(3’-indolyl)-isoxazoles, nortopsentin analogues. Bioorg. Med. Chem. 2010, 18, 4524–4529. [Google Scholar] [CrossRef]
- Karabasanagouda, T.; Adhikari, A.V.; Girisha, M. Synthesis of some new pyrazolines and isoxazoles carrying 4-methylthiophenyl moiety as potential analgesic and anti-inflammatory agents. Indian J. Chem. 2009, 48B, 430–437. [Google Scholar]
- Lee, Y.S.; Park, S.M.; Kim, B.H. Synthesis of 5-isoxazol-5-yl-2′-deoxyuridines exhibiting antiviral activity against HSV and several RNA viruses. Bioorg. Med. Chem. Lett. 2009, 19, 1126–1133. [Google Scholar] [CrossRef] [PubMed]
- Kano, H.; Adachi, I.; Kido, R.; Hirose, K. Isoxazoles. XVIII. Synthesis and Pharmacological Properties of 5-Aminoalkyl- and 3-Aminoalkylisoxazoles and Related Derivatives. J. Med. Chem. 1967, 10, 411–418. [Google Scholar] [CrossRef]
- Gu, Y. Multicomponent reactions in unconventional solvents: State of the art. Green Chem. 2012, 14, 2091–2128. [Google Scholar] [CrossRef]
- Kreysa, G.; Ota, K.; Savinell, R.F. Encyclopedia of Applied Electrochemistry; Springer: New York, NY, USA, 2014. [Google Scholar]
- Zang, W.; Yi, W.-B. Pot, Atom and Step Economy (PASE) Synthesis; Springer International Publishing: Cham, Switzerland, 2019; pp. 1–4. [Google Scholar]
- Elinson, M.N.; Dorofeev, A.S.; Nasybullin, R.F.; Fedukovich, S.K.; Nikihin, G.I. Electrocatalytic tandem Knoevenagel–Michael reaction of 3-methyl-2-pyrazolin-5-ones, aryl aldehydes and cyano-functionalized C–H acids: Facile and convenient multicomponent way to substituted 3-(5-hydroxy-3-methylpyrazol-4-yl)-3-arylpropionitriles. Electrochim. Acta 2008, 53, 5033–5038. [Google Scholar] [CrossRef]
- Elinson, M.N.; Nasybullin, R.F.; Nikihin, G.I. Electrocatalytic Fast and Efficient Multicomponent Approach to Medicinally Relevant (2-Amino-4H-chromen-4-yl)phosphonate Scaffold. Heteroatom. Chem. 2013, 24, 398–403. [Google Scholar] [CrossRef]
- Patai, S.; Israeli, Y. The kinetics and mechanisms of carbonyl–methylene condensations. Part VII. The reaction of malononitrile with aromatic aldehydes in ethanol. J. Chem. Soc. 1960, 2025–2030. [Google Scholar] [CrossRef]
- LeadFinder, Biomoltech, Inc. Available online: http://www.biomoltech.com/ (accessed on 22 April 2020).
- Stroganov, O.V.; Novikov, F.N.; Stroylov, V.S.; Kulkov, V.; Chilov, G.G. Lead Finder: An Approach To Improve Accuracy of Protein−Ligand Docking, Binding Energy Estimation, and Virtual Screening. J. Chem. Inf. Model. 2008, 48, 2371–2385. [Google Scholar] [CrossRef]
- Brennan, F.H.; Gordon, R.; Lao, H.W.; Biggins, P.J.; Taylor, S.M.; Franklin, R.J.; Woodruff, T.M.; Ruitenberg, M.J. The Complement Receptor C5aR Controls Acute Inflammation and Astrogliosis following Spinal Cord Injury. J. Neurosci. Res. 2015, 35, 6517–6531. [Google Scholar] [CrossRef] [Green Version]
- Lim, J.; Iyer, A.; Suen, J.Y.; Seow, V.; Reid, R.C.; Brown, L.; Fairlie, D.P. C5aR and C3aR antagonists each inhibit diet-induced obesity, metabolic dysfunction, and adipocyte and macrophage signaling. FASEB J. 2013, 27, 822–831. [Google Scholar] [CrossRef]
- Markiewski, M.M.; DeAngelis, R.A.; Benencia, F.; Ricklin-Lichtsteiner, S.K.; Koutoulaki, A.; Gerard, C.; Coukos, G.; Lambris, J.D. Modulation of the antitumor immune response by complement. Nat. Immunol. 2008, 9, 1225–1235. [Google Scholar] [CrossRef] [Green Version]
- Seow, V.; Lim, J.; Cotterell, A.; Yau, M.-K.; Xu, W.; Lohman, R.J.; Kok, W.M.; Stoermer, M.J.; Sweet, M.J.; Reid, R.C.; et al. Receptor residence time trumps drug-likeness and oral bioavailability in determining efficacy of complement C5a antagonists. Sci. Rep. 2016, 6, 24575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ko, T.Y.; Youn, S.W. Cooperative Indium(III)/Silver(I) System for Oxidative Coupling/Annulation of 1,3-Dicarbonyls and Styrenes: Construction of Five-Membered Heterocycles. Adv. Synth. Catal. 2016, 358, 1934–1941. [Google Scholar] [CrossRef]
- Griesbeck, A.G.; Franke, M.; Neudörfl, J.; Kotaka, H. Photocycloaddition of aromatic and aliphatic aldehydes to isoxazoles: Cycloaddition reactivity and stability studies. Beilstein J. Org. Chem. 2011, 7, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Bruker. APEX-III; Bruker AXS Inc.: Madison, WI, USA, 2018.
- Krause, L.; Herbst-Irmer, R.; Sheldrick, G.M.; Stalke, D. Comparison of silver and molybdenum microfocus X-ray sources for single-crystal structure determination. J. Appl. Cryst. 2015, 48, 3–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheldrick, G.M. SHELXT - Integrated space-group and crystal-structure determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar] [CrossRef]
- Liu, H.; Kim, H.R.; Deepak, R.N.V.K.; Wang, L.; Chung, K.Y.; Fan, H.; Wei, Z.; Zhang, C. Orthosteric and allosteric action of the C5a receptor antagonists. Nat. Struct. Mol. Biol. 2018, 25, 472–481. [Google Scholar] [CrossRef]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ryzhkova, Y.E.; Ryzhkov, F.V.; Elinson, M.N. 3-(4-Bromophenyl)-4-{[3-hydroxy-6-(hydroxymethyl)-4-oxo-4H-pyran-2-yl](m-tolyl)methyl}isoxazol-5(2H)-one. Molbank 2020, 2020, M1135. https://doi.org/10.3390/M1135
Ryzhkova YE, Ryzhkov FV, Elinson MN. 3-(4-Bromophenyl)-4-{[3-hydroxy-6-(hydroxymethyl)-4-oxo-4H-pyran-2-yl](m-tolyl)methyl}isoxazol-5(2H)-one. Molbank. 2020; 2020(2):M1135. https://doi.org/10.3390/M1135
Chicago/Turabian StyleRyzhkova, Yuliya E., Fedor V. Ryzhkov, and Michail N. Elinson. 2020. "3-(4-Bromophenyl)-4-{[3-hydroxy-6-(hydroxymethyl)-4-oxo-4H-pyran-2-yl](m-tolyl)methyl}isoxazol-5(2H)-one" Molbank 2020, no. 2: M1135. https://doi.org/10.3390/M1135
APA StyleRyzhkova, Y. E., Ryzhkov, F. V., & Elinson, M. N. (2020). 3-(4-Bromophenyl)-4-{[3-hydroxy-6-(hydroxymethyl)-4-oxo-4H-pyran-2-yl](m-tolyl)methyl}isoxazol-5(2H)-one. Molbank, 2020(2), M1135. https://doi.org/10.3390/M1135