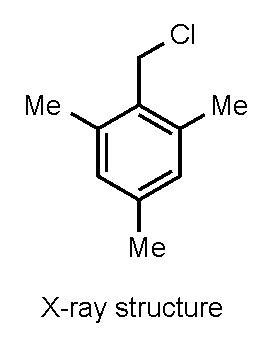
2,4,6-Trimethylbenzyl Chloride (α2-Chloroisodurene)
Abstract
1. Introduction
2. Results
3. Experimental
Author Contributions
Funding
Conflicts of Interest
References
- Sommelet, M. Sur une méthode de synthèse du chlorure de benzyle et de ses homologues. C. R. Hebd. Seances Acad. Sci. 1913, 157, 1443–1445. [Google Scholar]
- Hoch, J. Synthèse de quelques dérivés des acides arylacétique et β-arylpropionique. C. R. Hebd. Seances Acad. Sci. 1931, 192, 1464–1466. [Google Scholar]
- Vavon, G.; Bolle, J. Condensation des carbures benzéniques avec l’éther chlorométhylique. Méthode d’alcoylation des noyaux aromatiques. C. R. Hebd. Seances Acad. Sci. 1937, 204, 1826–1828. [Google Scholar]
- Vavon, G.; Bolle, J.; Calin, J. Condensation des éthers chloro et bromométhyliques sur les noyaux aromatiques. Bull. Soc. Chim. Fr. 1939, 6, 1025–1033. [Google Scholar]
- Nauta, W.T.; Dienske, J.W. Über einge mesitylen-derivate. Bildung eines aethers aus chlorid und methylalkohol. Recl. Trav. Chim. Pays-Bas 1936, 55, 1000–1006. [Google Scholar] [CrossRef]
- Fuson, R.C.; Rabjohn, N. Mesitylacetic acid. Org. Synth. 1945, 25, 65–68. [Google Scholar] [CrossRef]
- Mannion, J.J.; Wang, T.S. An infrared study of the CH2Cl group in benzyl chloride and derivatives. Spectrochim. Acta 1964, 20, 45–49. [Google Scholar] [CrossRef]
- Yakubov, A.P.; Tsyganov, D.V.; Belen’kii, L.I.; Bogdanov, V.S.; Ugrak, B.I.; Krayushkin, M.M. Unusual effects of steric hindrances in NMR spectra of o,o’-dialkylsubstituted benzylidene dichlorides. Tetrahedron 1991, 47, 5237–5244. [Google Scholar] [CrossRef]
- Dewar, M.J.S.; Herr, M.L. Chlorine NQR spectra of some benzyl chlorides, allyl chlorides, and chlorophosphines. Tetrahedron 1971, 27, 2377–2383. [Google Scholar] [CrossRef]
- Motsch, S.; Schütz, C.; Huy, P.H. Systematic evaluation of sulfoxides as catalysts in nucleophilic substitutions of alcohols. Eur. J. Org. Chem. 2018, 4541–4547. [Google Scholar] [CrossRef]
- Nayak, S.K.; Sathishkumar, R.; Guru Row, T.N. Directing role of functional groups in selective generation of C–H...p interactions: In situ cryo-crystallographic studies on benzyl derivatives. CrystEngComm 2010, 12, 3112–3118. [Google Scholar] [CrossRef]
- Basaran, R.; Dou, S.-Q.; Weiss, A. On the persistence of molecular symmetry in the solid state and the role of molecular group dipole moments. Centrosymmetry in bis(chloromethyl)benzenes. Ber. Bunsenges. Phys. Chem. 1992, 96, 1688–1698. [Google Scholar] [CrossRef]
- Sanders, M.B.; Leon, D.; Ndichie, E.I.; Chan, B.C. 1,3-Bis(chloromethyl)benzene. Acta Crystallogr. Sect. E 2013, 69, o1150. [Google Scholar] [CrossRef] [PubMed]
- Bryan, J.C.; Burnett, M.N.; Sachleben, R.A. 1,3,5-Tris(chloromethyl)benzene. Acta Crystallogr. Sect. C 2000, 56, e167. [Google Scholar] [CrossRef] [PubMed]
- Bart, S.C.; Heinemann, F.W.; Anthon, C.; Hauser, C.; Meyer, K. A new tripodal ligand system with steric and electronic modularity for uranium coordination chemistry. Inorg. Chem. 2009, 48, 9419–9426. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. A short history of SHELXL. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
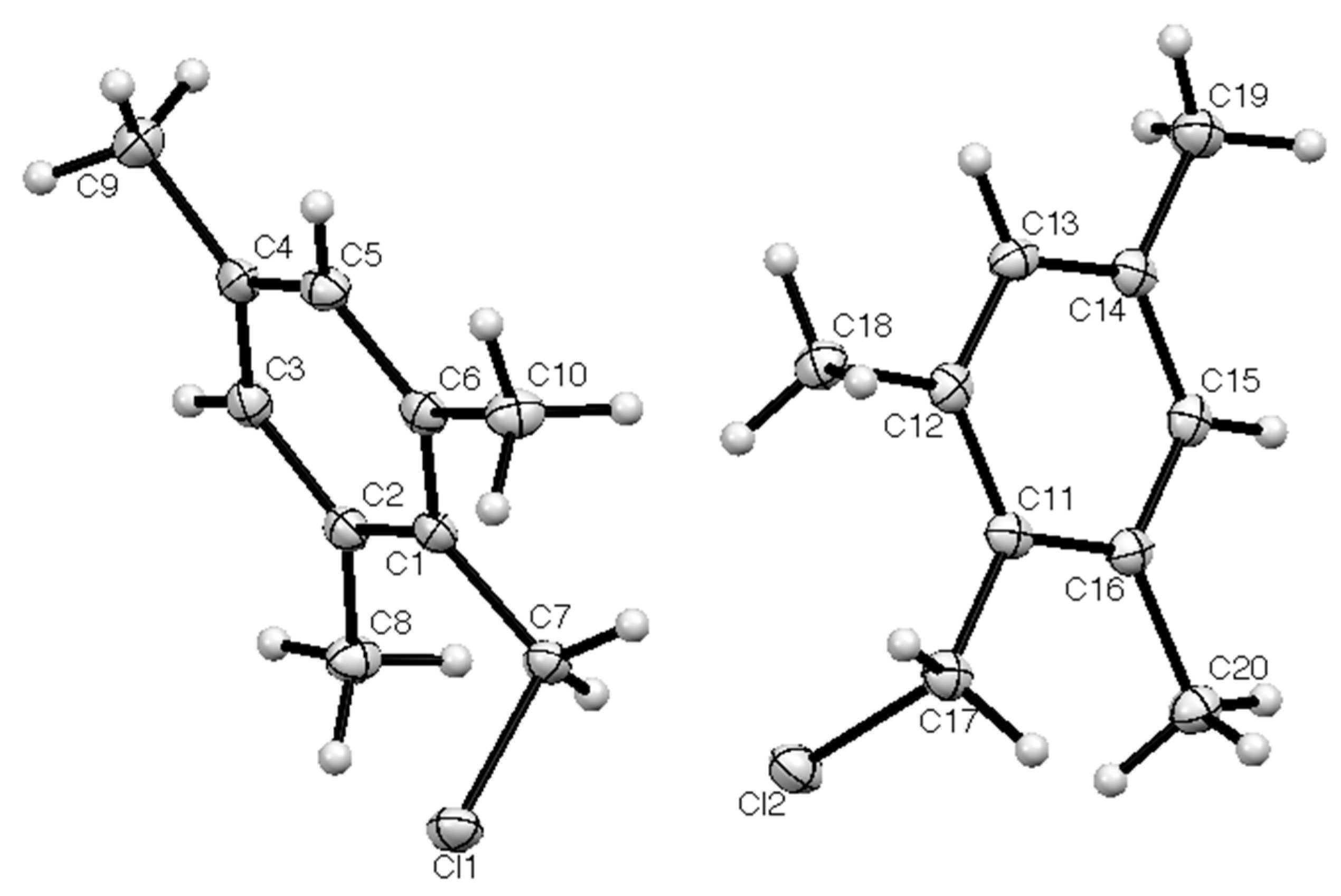
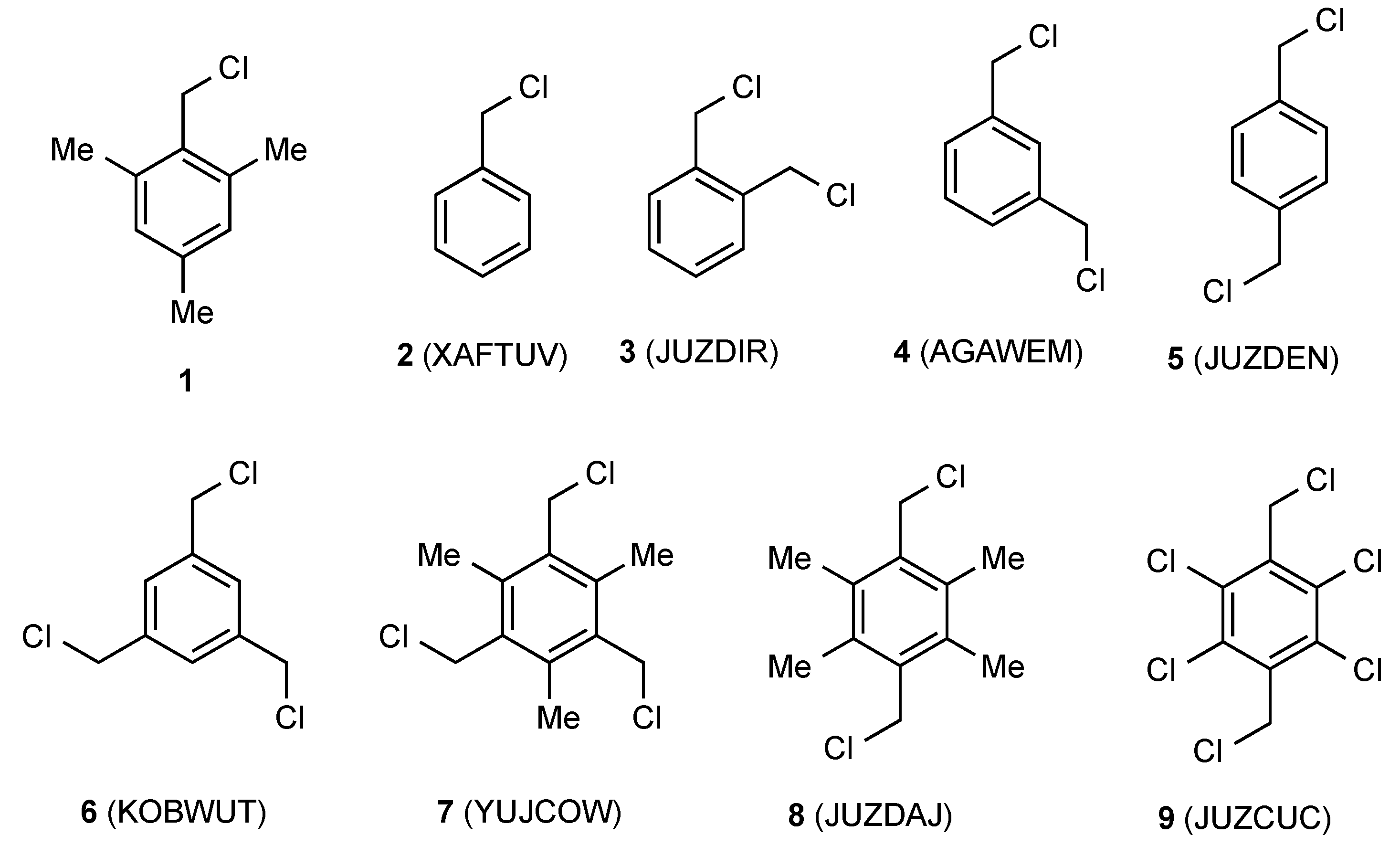
| Compd | Length C–CH2 (Å) | Length CH2–Cl (Å) | Angle C–CH2–Cl° | Torsion Angle Ring/CH2–Cl° | Reference |
|---|---|---|---|---|---|
| 1 | 1.500(2) | 1.831(1) | 112.32(9) | 85.50 | this work |
| * | 1.500(2) | 1.829(1) | 111.34(9) | 85.51 | |
| 2 | 1.492(3) | 1.801(3) | 111.5(2) | 78.38 | [11] |
| 3 | 1.498(6) | 1.814(5) | 111.5(3) | 81.03 | [12] |
| 4 | 1.501(2) | 1.811(1) | 109.98(8) | 78.49 | [13] |
| 1.500(2) | 1.815(1) | 109.92(8) | 83.52 | ||
| 5 | 1.494(3) | 1.805(3) | 111.0(2) | 86.06 | [12] |
| 6 | 1.498(3) | 1.806(2) | 110.6(2) | 85.98 | [14] |
| 1.494(3) | 1.804(3) | 110.5(2) | 84.29 | ||
| 1.496(3) | 1.793(2) | 110.8(1) | 82.11 | ||
| * | 1.495(3) | 1.805(2) | 110.4(2) | 77.48 | |
| * | 1.495(3) | 1.804(2) | 111.2(1) | 68.88 | |
| * | 1.501(3) | 1.790(2) | 110.1(1) | 82.60 | |
| 7 | 1.501(3) | 1.814(3) | 111.3(2) | 84.31 | [15] |
| 1.502(3) | 1.815(2) | 111.3(2) | 86.53 | ||
| 1.504(5) | 1.810(3) | 109.8(2) | 83.13 | ||
| 8 | 1.493(5) | 1.817(5) | 111.3(3) | 88.55 | [12] |
| 9 | 1.497(3) | 1.796(3) | 110.6(2) | 81.11 | [12] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aitken, R.A.; Slawin, A.M.Z. 2,4,6-Trimethylbenzyl Chloride (α2-Chloroisodurene). Molbank 2020, 2020, M1156. https://doi.org/10.3390/M1156
Aitken RA, Slawin AMZ. 2,4,6-Trimethylbenzyl Chloride (α2-Chloroisodurene). Molbank. 2020; 2020(3):M1156. https://doi.org/10.3390/M1156
Chicago/Turabian StyleAitken, R. Alan, and Alexandra M. Z. Slawin. 2020. "2,4,6-Trimethylbenzyl Chloride (α2-Chloroisodurene)" Molbank 2020, no. 3: M1156. https://doi.org/10.3390/M1156
APA StyleAitken, R. A., & Slawin, A. M. Z. (2020). 2,4,6-Trimethylbenzyl Chloride (α2-Chloroisodurene). Molbank, 2020(3), M1156. https://doi.org/10.3390/M1156