(7E)-3-(4-Methoxyphenyl)-7-[(4-methoxyphenyl)methylidene]-4,5,6,7-tetrahydro-3aH-indazole
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General
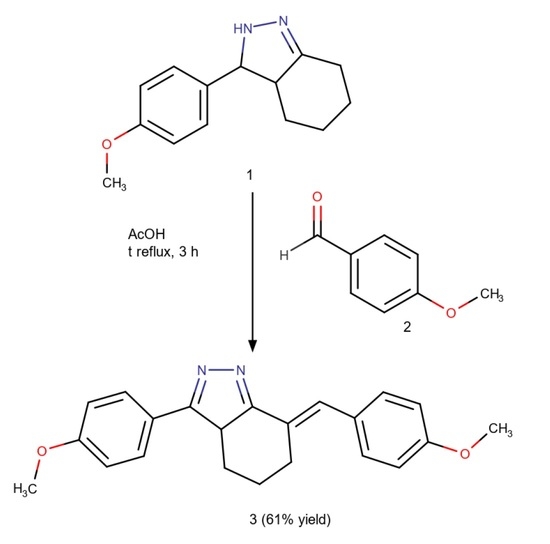
3.2. Synthesis of (7E)-3-(4-Methoxyphenyl)-7-[(4-methoxyphenyl)methylidene]-4,5,6,7-tetrahydro-3aH-indazole (3)
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, S.G.; Liang, C.G.; Zhang, W.H. Recent Advances in Indazole-Containing Derivatives: Synthesis and Biological Perspectives. Molecules 2018, 23, 2783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaikwad, D.D.; Warad, K.D.; Chapolikar, A.D.; Devkate, C.G.; Tayade, A.P.; Pawar, R.P.; Domb, A.J. Synthesis of indazole motifs and their medicinal importan ce: An overview. Eur. J. Med. Chem. 2015, 90, 707–731. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, A.; Chakraborty, A.K.; Upmanyu, N.; Singh, A. Recent Progress in Chemistry and Biology of Indazole and its Derivatives: A Brief Review. Austin. J. Anal. Pharm. Chem. 2016, 3, 1076. [Google Scholar]
- Thangadurai, A.; Minu, M.; Wakode, S.; Agrawal, S.; Narasimhan, B. Indazole: A medicinally important heterocyclic moiety. Med. Chem. Res. 2012, 21, 1509–1523. [Google Scholar] [CrossRef]
- Ismail, Z.H. The use of a-enone derivative in the preparation of some new heterocyclic compounds with expected biological and antitumor activities. Adv. Environ. Biol. 2013, 7, 1049–1057. [Google Scholar]
- Minu, M.; Thangadurai, A.; Wakode, S.R.; Agrawal, S.S.; Narasimhan, B. Synthesis, antimicrobial activity and QSAR studies of new 2,3-disubstituted-3,3a,4,5,6,7-hexahydro-2H-indazoles. Bioorg. Med. Chem. Let. 2009, 19, 2960–2964. [Google Scholar] [CrossRef] [PubMed]
- Bayomi, S.M.; El-Kashef, H.A.; El-Ashmawy, M.B.; Nasr, N.A.; El-Sherbeny, M.A.; Badria, A. Synthesis and biological evaluation of new curcumin derivatives as antioxidant and antitumor agents. Med. Chem. Res. 2013, 22, 1147–1162. [Google Scholar] [CrossRef]
- Silverstein, R.M.; Webster, F.X.; Kiemle, D.J. Spectrometric Identification of Organic Compounds, 7th ed.; John Wiley & Sons, Inc.: New York, NY, USA, 2005. [Google Scholar]
- MarvinSketch 20.8.0. Chemaxon Ltd. 1998–2020. Available online: http://www.chemaxon.com (accessed on 28 September 2020).
- Nájera, C.; Sansano, J.M.; Yus, M. 1,3-Dipolar Cycloadditions of azomethine imines. Org. Biomol. Chem. 2015, 13, 8596–8636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nuriev, V.N.; Vatsadze, I.A.; Sviridenkova, N.V.; Vatsadze, S.Z. Synthesis of 3,7-Disubstituted Hexahydro and Tetrahydro-2H-indazoles from Cross-Conjugated Dienones. Russ. J. Org. Chem. 2016, 52, 389–396. [Google Scholar] [CrossRef]
- Hudiyono, S.; Hanafi, M.; Yanuar, A. Synthesis and COX-2 Inhibitory Activity of 4-[(E)-2-(4-Oxo-3-phenyl-3,4-dihydroquinazolin-2-yl)ethenyl]benzene-1-sulfonamide and Its Analogs. Pharmaceuticals 2012, 5, 1282–1290. [Google Scholar] [PubMed]
- Sridhar, R.; Takei, H.; Syed, R.; Kobayashi, I.S.; Hui, L.B.; Kamal, A.; Tenen, D.G.; Kobayashi, S.S. Styryl Quinazolinones as Potential Inducers of Myeloid Differentiation via Upregulation of C/EBPα. Molecules 2018, 23, 1938. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hariyanti, H.; Yanuar, A.; Kusmardi, K.; Hayun, H. (7E)-3-(4-Methoxyphenyl)-7-[(4-methoxyphenyl)methylidene]-4,5,6,7-tetrahydro-3aH-indazole. Molbank 2020, 2020, M1162. https://doi.org/10.3390/M1162
Hariyanti H, Yanuar A, Kusmardi K, Hayun H. (7E)-3-(4-Methoxyphenyl)-7-[(4-methoxyphenyl)methylidene]-4,5,6,7-tetrahydro-3aH-indazole. Molbank. 2020; 2020(4):M1162. https://doi.org/10.3390/M1162
Chicago/Turabian StyleHariyanti, Hariyanti, Arry Yanuar, Kusmardi Kusmardi, and Hayun Hayun. 2020. "(7E)-3-(4-Methoxyphenyl)-7-[(4-methoxyphenyl)methylidene]-4,5,6,7-tetrahydro-3aH-indazole" Molbank 2020, no. 4: M1162. https://doi.org/10.3390/M1162
APA StyleHariyanti, H., Yanuar, A., Kusmardi, K., & Hayun, H. (2020). (7E)-3-(4-Methoxyphenyl)-7-[(4-methoxyphenyl)methylidene]-4,5,6,7-tetrahydro-3aH-indazole. Molbank, 2020(4), M1162. https://doi.org/10.3390/M1162