Abstract
The title compound, diethyl [(4-{(9H-carbazol-9-yl)methyl}-1H-1,2,3-triazol-1-yl)(benzamido)methyl]phosphonate, was synthesized with excellent yield and high regioselectivity through 1,3-dipolar cycloaddition reaction between the α-azido diethyl amino methylphosphonate and the heterocyclic alkyne, 9-(prop-2-yn-1-yl)-9H-carbazole. The cyclization reaction by “click chemistry” was carried out in a water/ethanol solvent mixture (50/50), in the presence of copper sulfate pentahydrate and catalytic sodium ascorbate. The characterization of the structure of the resulting 1,4-regioisomer was performed by 1D and 2D-NMR experiments, infrared spectroscopy, and elemental analysis.
1. Introduction
For several decades, in the face of microbial resistance to antibiotics, there has been a strong rush to synthesize organophosphorus compounds [1], which are considered to be new bioisosters of amino carboxylic acids. The succession of N–C–P bonds gives α-amino phosphonic acids important electronic properties, such as the ability to have an acid–base behavior, in order to form a network of specific hydrogen bonds, which is very important in biological processes [2]. Thus, important biological and environmental properties of heterocyclic phosphonic amino acids have been identified in the literature [3]. They proved their relevance in the design of new formulae for osteoporosis drugs [4], as antimicrobial and antioxidant agents [5,6], anticancer compounds [7], and antibacterial compounds [8], but also in the study of corrosion inhibition [9,10]. Thus, methods for the synthesis of phosphonic amino acids bearing a heterocycle in the alpha position have been described [11,12]. However, their triazolic analogs remain very limited [13]. That is why we have been interested in their preparation. Continuing our investigations on the synthesis of heterocyclic phosphonic [14], we described in this paper our results concerning the synthesis of a new biheterocyclic phosphonic amino ester, namely diethyl [(4-{(9H-carbazol-9-yl)methyl}-1H-1,2,3-triazol-1-yl)(benzamido)methyl]phosphonate. The approach adopted was to prepare, in a first step, the heterocyclic dipolarophile (2) by nucleophilic substitution of 9H-carbazole [15] on propargyl bromide. Next, we performed a 1,3-dipolar cycloaddition reaction by “click chemistry” [16,17] between diethyl (azido(benzamido)methyl)phosphonate (1) and 9-(prop-2-yn-1-yl)-9H-carbazole (2). The desired cycloadduct (3) was achieved regioselectively in high yield, and characterized by 1D-NMR (31P, 1H, 13C), 2D-NMR (1H-1H, 1H-13C), IR, and elemental analysis (Supplementary Materials).
2. Results and Discussion
The azide dipole (1) was prepared according to a six-step synthesis, adopting the approach of Elachqar et al. [14]. This strategy requires anhydrous and inert operating conditions. The last step to obtain the azide dipole (1) was the action of sodium azide on the α-bromo-α-aminomethyl N-benzoylated diethyl phosphonate. The overall yield of this resynthesis strategy is 85%.
The product 9-(prop-2-yn-1-yl)-9H-carbazole (2) (CAS No. (4282-77-3)), used in the cycloaddition reaction, was resynthesized in 80% yield at our laboratory, according to the approach described in the literature [15], by nucleophilic substitution of propargyl bromide with carbazole.
The cyclization reaction by click chemistry was carried out by condensation of dipolarophile (2) and dipole azide (1) in a water/ethanol solvent mixture (50/50), in the presence of copper sulfate pentahydrate and catalytic sodium ascorbate (Scheme 1). The biheterocyclic derivative of the phosphonic analog of glycine was obtained with an excellent yield (90%), as a white solid after chromatography on a silica gel column (ethyl acetate/hexane: 1/1) and recrystallization in an ether/hexane mixture. The chemical structure of the title compound was confirmed by 1D and 2D-NMR, IR, and elemental analysis.
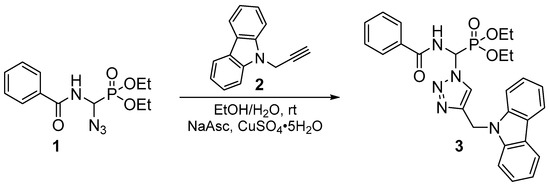
Scheme 1.
Synthetic route for compound (3).
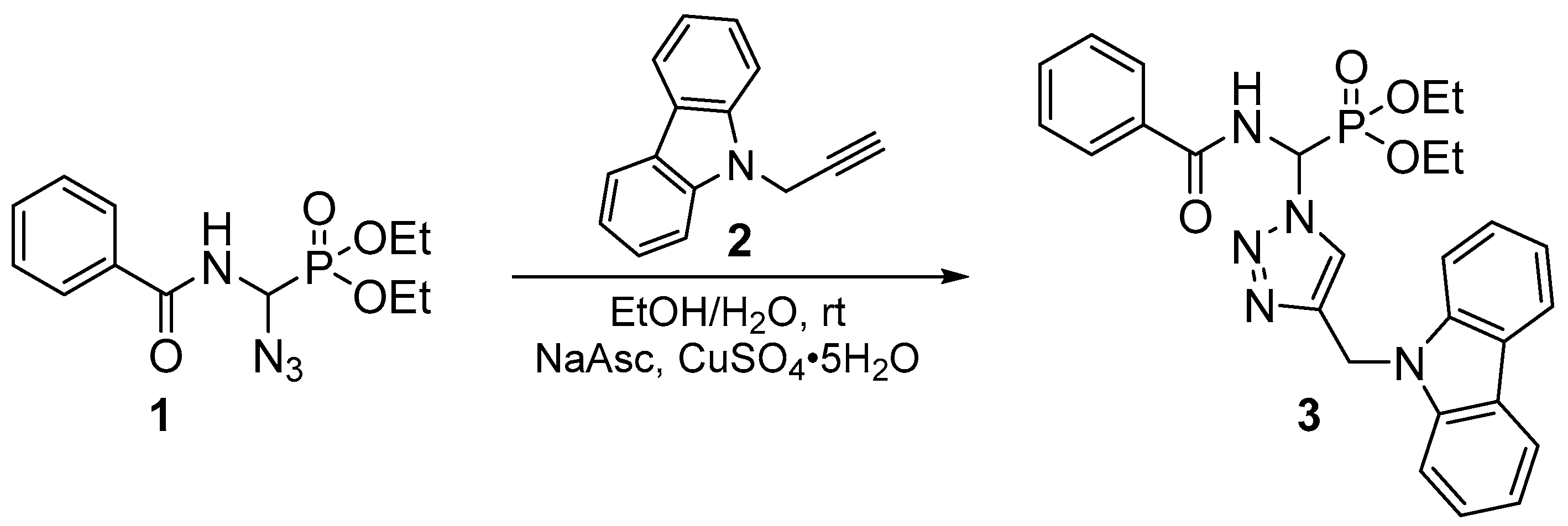
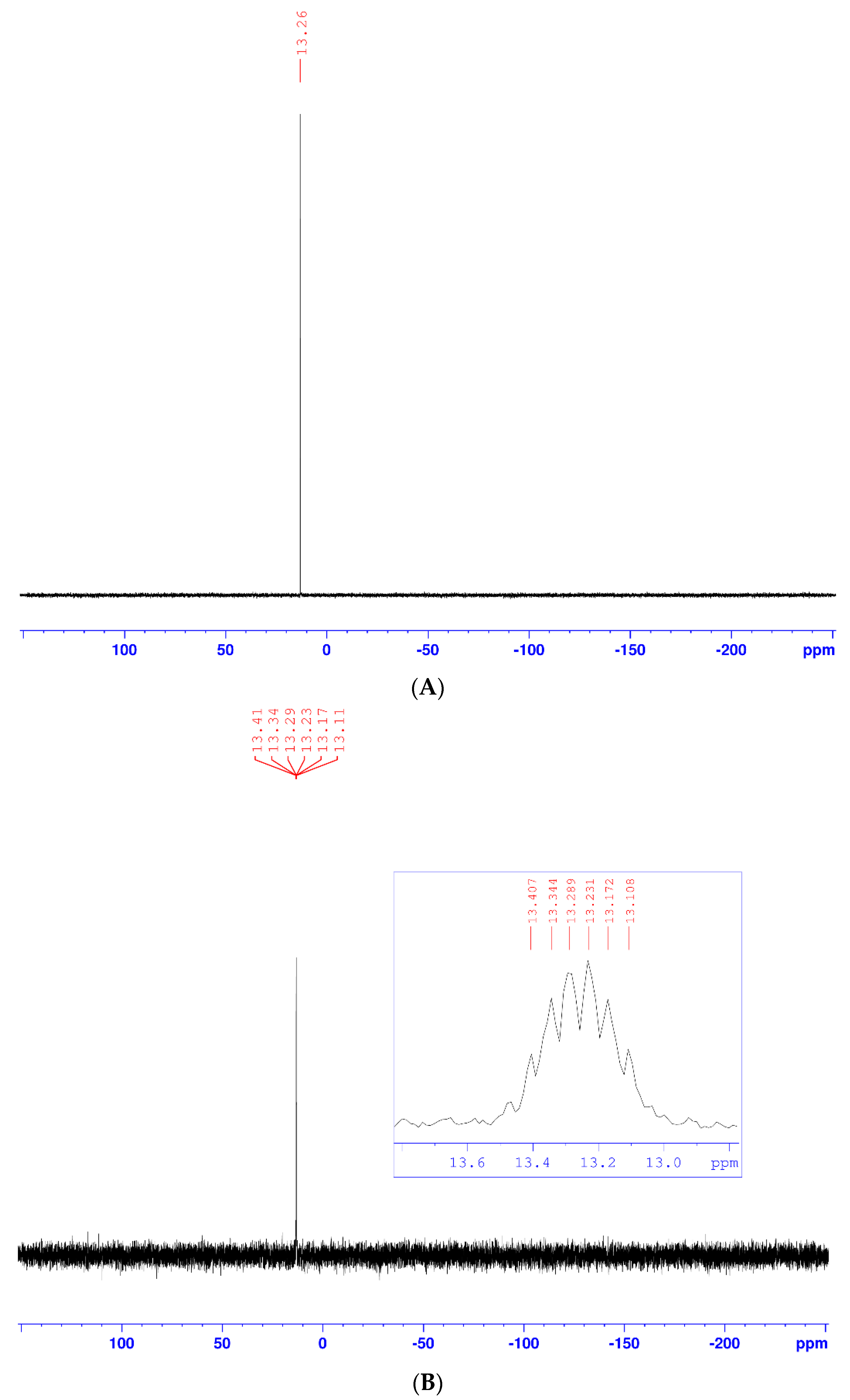
According to the Huisgen method [18] and previous work carried out in our research laboratory [19,20,21,22], the 1,3-dipolar cycloaddition reaction led to the formation of a regioisomeric mixture of 1,4- and 1,5-regioisomers, with a prevalence of the 1,4-regioisomer. However, under the conditions of click chemistry, depending on the nature of the metal ion used as a catalyst, the cycloaddition reactions selectively led to a single 1,4- or 1,5-regioisomer. Thus, in the presence of copper (II) ions [16], only the 1,4-regioisomer was obtained, whereas, in the presence of rhodium (I) [17], only the 1,5-regioisomer was obtained. For our part, during the cycloaddition reaction using copper (II) sulfate and sodium ascorbate, only the 1,4-regioisomer (3) was obtained, with an excellent yield (90%), as a white solid after chromatography on a silica gel column (ethyl acetate/hexane: 1/1), and recrystallization in an ether/hexane mixture. Its structure was established on the basis of decoupled and coupled 31P-NMR (Figure 1), 1D 1H and 13C-NMR (Figure 2 and Figure 3), homonuclear (1H-1H) and heteronuclear (1H-13C) 2D-NMR (Figure 4 and Figure 5), infrared spectroscopy (Figure 6), and elemental analysis. Therefore, the cycloadduct (3) exhibits, in the decoupled 31P-NMR spectrum (Figure 1A), a single signal at 13.26 ppm.
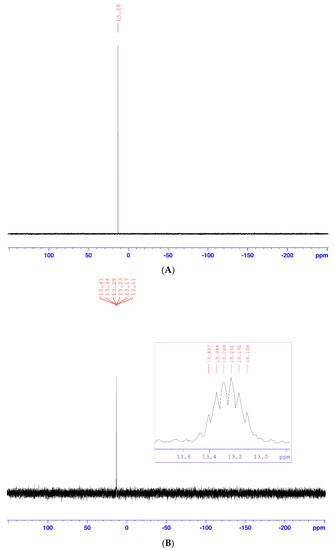
Figure 1.
(A) Decoupled 31P-NMR spectra of compound (3); (B) Coupled 31P-NMR spectra of compound (3).
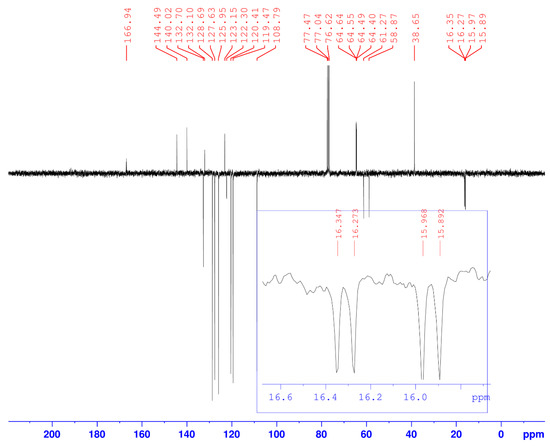
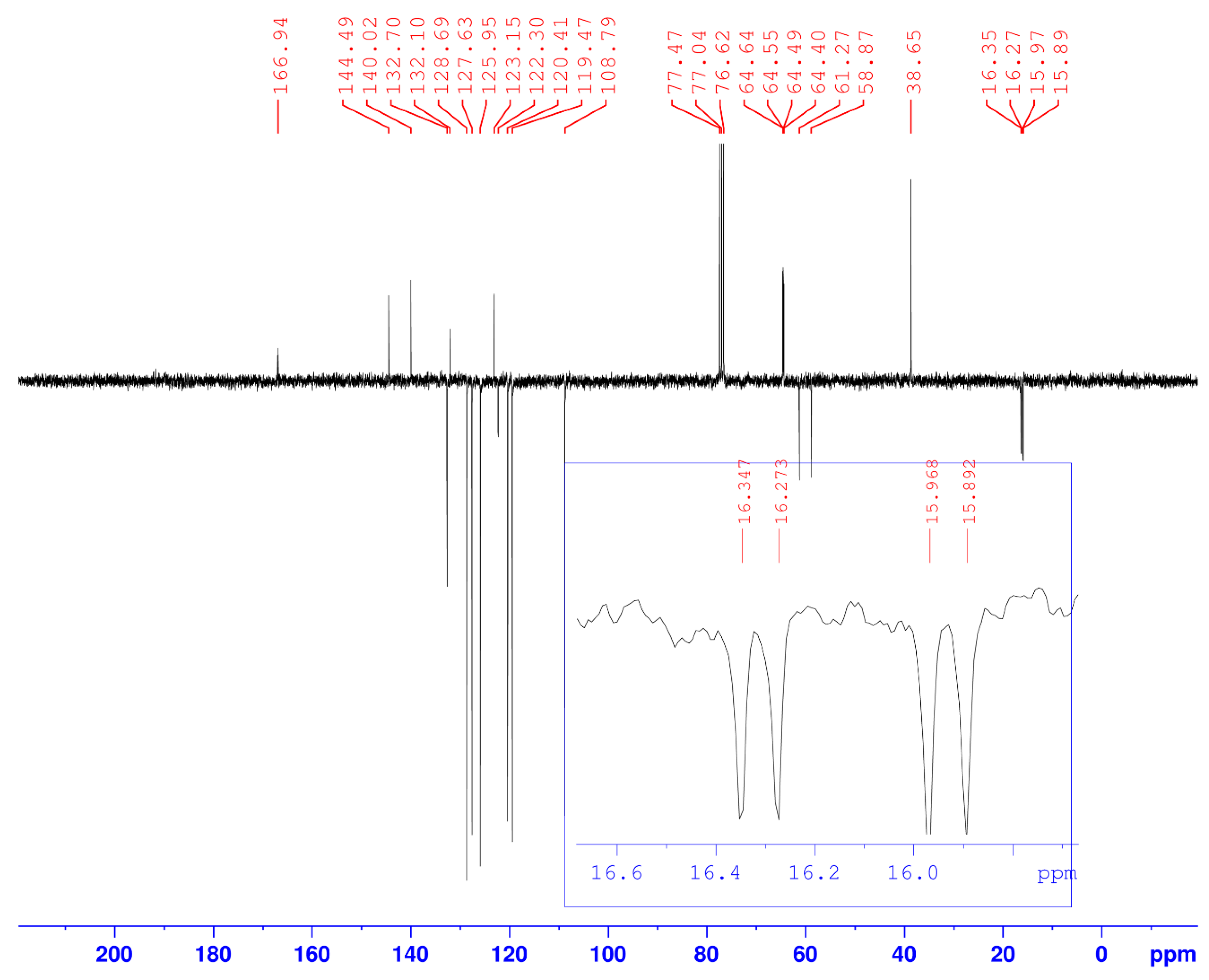
Figure 2.
13C-NMR spectrum of compound (3).
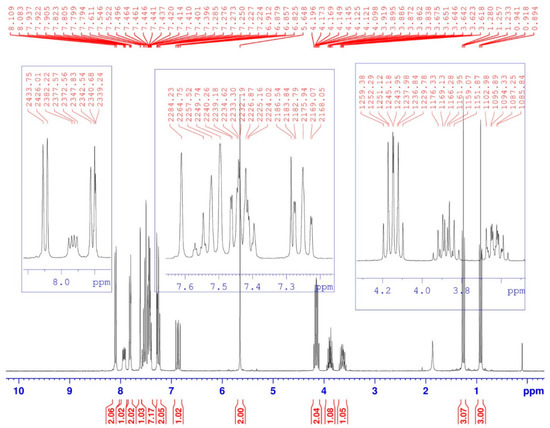
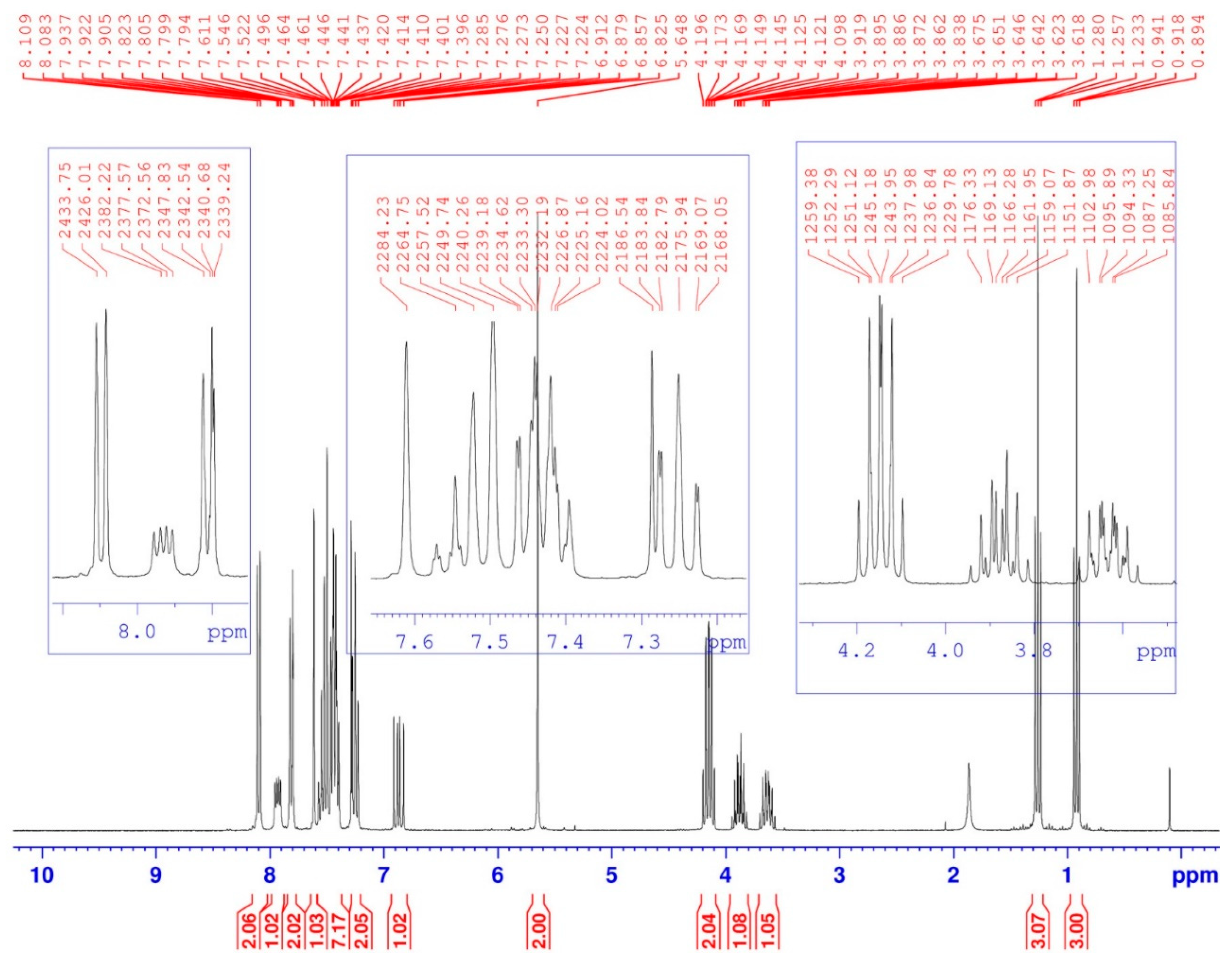
Figure 3.
1H-NMR spectrum of compound (3).
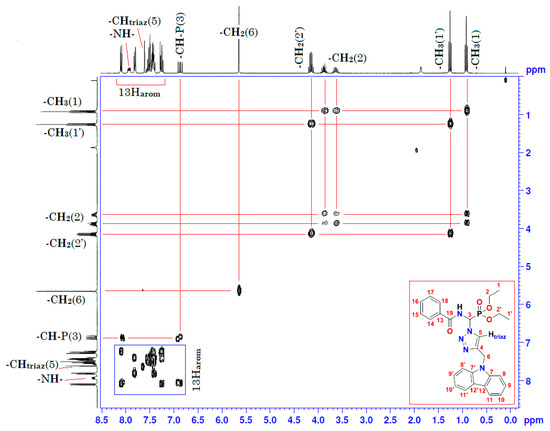
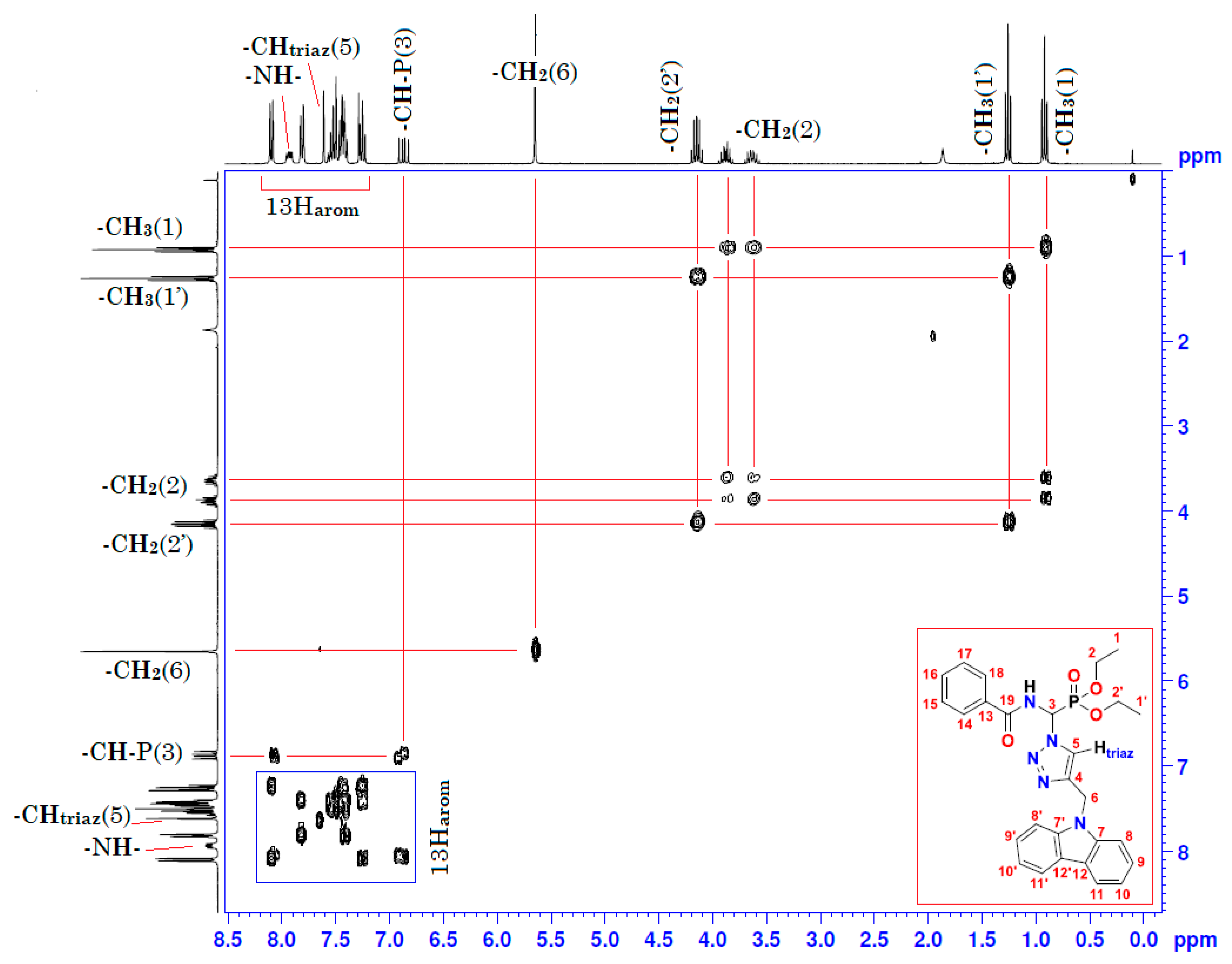
Figure 4.
Homonuclear 1H-1H 2D spectrum of compound (3).
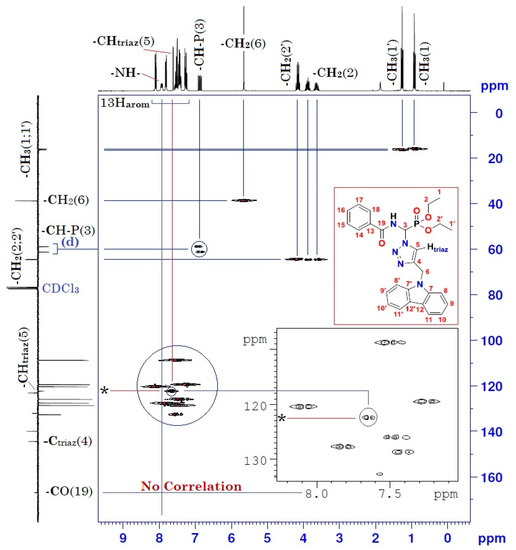
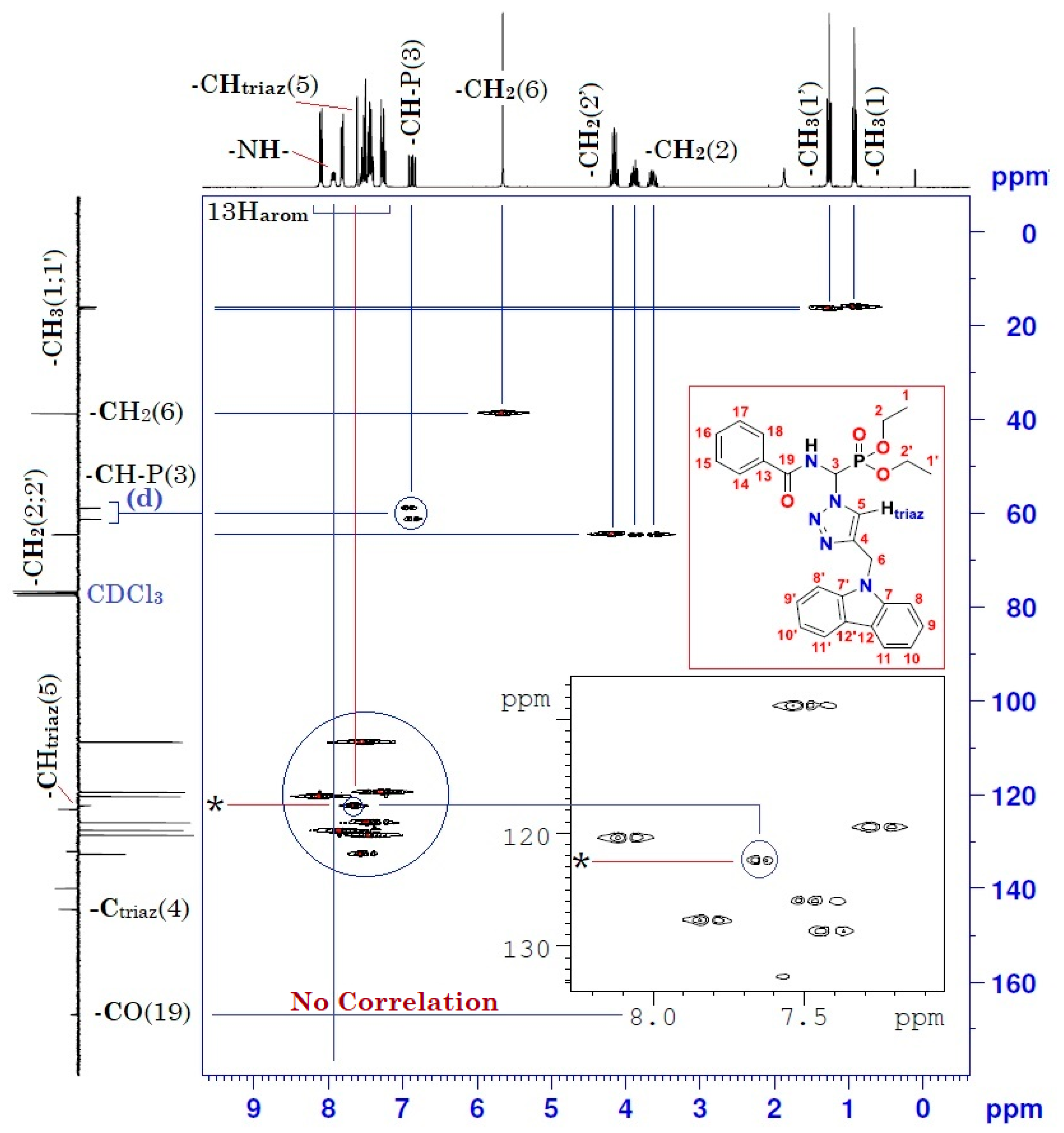
Figure 5.
Heteronuclear 1H-13C 2D spectrum of compound (3).
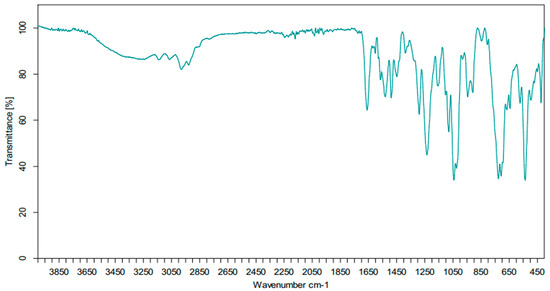
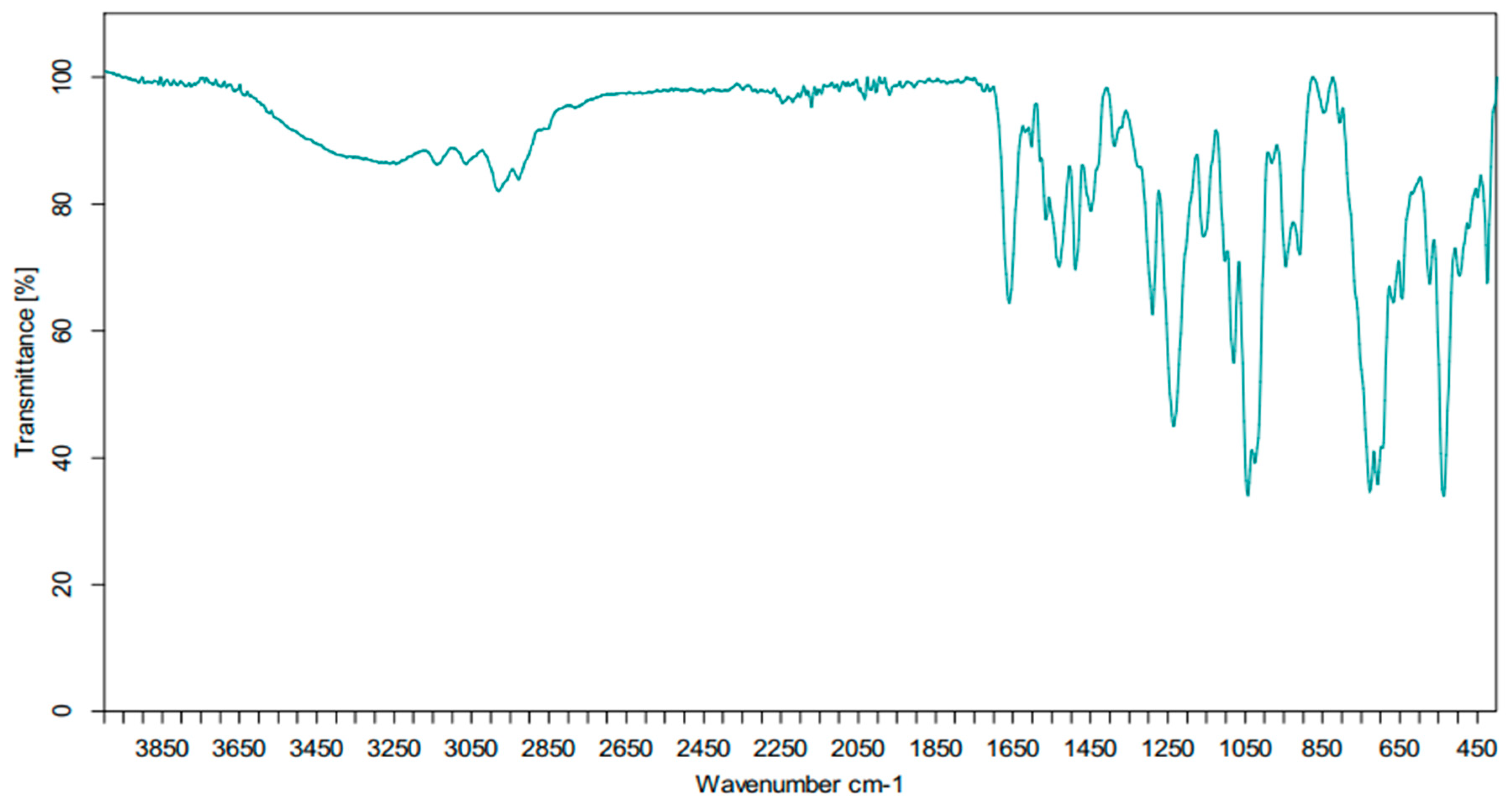
Figure 6.
IR spectrum of compound (3).
While it presents in the coupled 31P-NMR spectrum (Figure 1B) a single signal centered at 13.27 ppm, the spread of this signal shows that it is a multiplet, because of the coupling of phosphorus with neighboring carbons and hydrogens.
In addition, in the 13C-NMR spectrum (Figure 2), we note the coupling of phosphorus with the α carbon of the phosphonate (1JC-P = 181.12 Hz), and with the carbons of the two ethoxy groups which resonate as a doublet (2JC-P = 11.3 Hz and 3JC-P ~ 6 Hz).
While in 1H-NMR (Figure 3), we note that the hydrogen carried by the α carbon of the phosphonate group appears as a doublet split, due to coupling with both amidic hydrogen -NH and phosphorus 31P, 3JH-H = 10 Hz and 2JH-P = 15.4 Hz. While the two protons of the first ethoxy group OCH2CH3 resonate as a split quintuplet, due to coupling with both the three protons of the methyl group -CH3 and phosphorus 31P, 3JH-H = 7 Hz and 3JH-P = 1.5 Hz. Finally, the two OCHaHb protons of the other ethoxy group resonate as two split sextuplets because the two protons Ha and Hb are not magnetically equivalent. The coupling constants are identical to the previous ones.
Moreover, the analysis of the homonuclear 1H-1H 2D spectrum of compound (3) shows a perfect correlation between neighboring protons (Figure 4 and Table 1). It is important to specify that the two hydrogen atoms carried by the carbon atom -CH2- of the ethoxy group resonate as two split quintuplets, because the two protons Ha and Hb are not magnetically equivalent. This observation was confirmed in both mono- and heteronuclear 2D-NMR (Figure 4 and Figure 5). The definite assignment the chemical shifts of protons and carbons are shown in Table 1.

Table 1.
1H (300 MHz) and 13C (75 MHz) NMR spectral data for compound (3) in CDCl3, including results obtained by homonuclear 2D shift-correlated and heteronuclear 2D shift-correlated HMBC. Chemical shifts (δ in ppm) and coupling constants (J in Hz).
The interpretation of the heteronuclear 2D-NMR spectrum of the cycloadduct shows a perfect correlation, between protons and adjacent carbons on the one hand, and between protons and neighboring carbons on the other hand (Figure 5 and Table 1). In addition, the 2D heteronuclear spectrum allowed us to confirm the proposed structure of the cycloadduct. Thus, these results are in perfect agreement with the literature data on “click chemistry” which attributes the structure of the product to the 1,4-regioisomer. This structure is also assigned on the basis of literature data on chemical shifts of triazolic protons [23,24]. The chemical shift of the triazolic proton in position 5 (δH5) of the triazolic cycle is more deshielded than its counterpart in position 4 for the 1,5-regioisomer (δH5 > δH4). Its signal is generally between 8 and 8.5 ppm. Thus, according to the 1H-13C 2D heteronuclear spectrum of compound (3), the triazole proton at 7.61 ppm correlates perfectly with its adjacent carbon, which resonates in the 13C-NMR at 122.30 ppm (Figure 5 and Table 1). The value of the chemical shift of the triazole proton in position 5 of our compound (3) is 7.61 ppm. This value is lower than the values described in the literature. This slight shielding can be explained by the effect of magnetic anisotropy due to the resonance caused by the delocalization of π-electrons from the carbazole ring. This resonance creates an induced magnetic field B (Bind) which opposes the external magnetic field B0 of the NMR apparatus, giving rise to a lower effective magnetic field B (Beff), and resulting in the shielding of all protons in the space of the two cones opposed by the summit, which lies in the middle of the horizontal plane of the carbazole ring. Consequently, the triazolic proton in position 5 may be found in this shielding area, which explains the value below 8 ppm (δH5 = 7.61 < 8 ppm). Conversely, the protons of the two benzene rings of carbazole at 8, 8′, 11, and 11′ are in a horizontal plane, with an effective Beff magnetic field resulting from the sum of B0 and Bind. The latter is the origin of the deshielding observed for its protons.
In addition, we obtained the infrared spectrum of the compound (3) (Figure 6). The assignments of the bands observed and the wavenumbers relative intensities are listed in Table 2.

Table 2.
Bands assignments (cm−1) in the IR spectrum of compound (3).
The infrared spectrum of the cycloadduct (3) shows:
- ◾
- Two bands in the range of 3050–3100 cm−1, corresponding to the elongation vibrations of ν(Csp2-H).
- ◾
- Two bands in the range of 2900–2950 cm−1, corresponding to the elongation vibrations of ν(Csp3-H).
- ◾
- One intense band around 1650 cm−1, attributable to the C=O function.
- ◾
- A strong band at 1250 cm−1, corresponding to the valence vibrations of the ν(P=O) function.
- ◾
- A medium band at 1050 cm−1, associated with the valence vibrations of the ν(P–O–C) link.
- ◾
- The observation of the bands around 850 and 900 corresponds to the deformation vibrations of ν(N–H).
All these interpretations allowed us to propose for the cycloadduct (3), the nomenclature of the 1,4-regioisomer, which is diethyl [(4-{(9H-carbazol-9-yl)methyl}-1H-1,2,3-triazol-1-yl)(benzamido)methyl]phosphonate.
3. Materials and Methods
All solvents were purified following the standard techniques and commercial reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA). Melting point was determined with an electrothermal melting point apparatus and was uncorrected. NMR spectra (1H and 13C) were recorded on a Bruker AM 300 spectrometer (operating at 300.13 MHz for 1H, at 75.47 MHz for 13C) (Bruker Analytische Messtechnik GmbH, Rheinstetten, Germany). NMR data are listed in ppm and are reported relative to tetramethylsilane (1H, 13C); residual solvent peaks being used as an internal standard. All reactions were followed by thin-layer chromatography (TLC). TLC analyses were carried out on 0.25 mm thick precoated silica gel plates (Merck Fertigplatten Kieselgel 60F254) and spots were visualized under UV light or by exposure to vaporized iodine. The FT-IR spectrum was recorded in KBr pellet on a Bruker Vertex 70 FTIR spectrometer. Elemental analysis was performed with a Flash 2000 EA 1112, Thermo Fisher Scientific-Elemental Analyzer (CNRST, Rabat, Morocco).
To a solution of 2.2 mmol of azide (1) and 2.2 mmol of alkyne (2) in 10 mL of an ethanol–water mixture (1/1), 0.05 equivalent of copper sulfate pentahydrate (CuSO4·5H2O) was used as catalyst with 0.1 equivalent of sodium ascorbate (NaAsc). The reaction mixture was stirred for 24 h at room temperature. As soon as the reaction was completed, the precipitate formed was filtered, and the solvent was evaporated under reduced pressure. The resulting crude was washed with water and extracted with methylene chloride. The organic layer was dried over sodium sulfate (Na2SO4), and the solvent was removed under reduced pressure. The resulting oil was purified by column chromatography of silica gel (ethyl acetate/hexane).
Diethyl [(4-{(9H-carbazol-9-yl)methyl}-1H-1,2,3-triazol-1-yl)(benzamido)methyl]phosphonate (3). Yield = 90% (White solid); Rf = 0.4 (ether/ methanol 5%); m.p. = 226–228 °C. 1H-NMR (CDCl3, δH ppm): 0.92 (t, 3H, -CH2-CH3, 3J = 7 Hz), 1.26 (t, 3H, -CH2-CH3, 3J = 7 Hz), 3.62–3.67 (m, 1H, -OCHaHb-CH3), 3.84–3.92 (m, 1H, -OCHaHb-CH3), 4.1–4.2 (m, 2H, (P-O-CH2-CH3)), 5.65 (s, 2H, triaz-CH2-carbaz), 6.83–6.91 (dd, 1H, NH-CH-P, 3JH-H = 10 Hz, 2JH-P = 15.4 Hz,), 7.22–7.27 (m, 2Harom(carbaz)), 7.40–7.55 (m, 7H, 5Harom(Ph) + 2Harom(carbaz)), 7.61 (s, 1H, -CHtriaz), 7.79–7.82 (d, 2Harom(carbaz), J = 8 Hz), 7.90–7.95 (dd, 1H, NH-CH-P, 3JH-H = 9.66 Hz, 3JH-P = 5.01 Hz), 8.08–8.11 (d, 2Harom(carbaz), J = 8 Hz).
13C-NMR (CDCl3, δC ppm): 15.89–15.97 (d, 1C, -PO-CH2-CH3, 3JC-P = 5.7 Hz), 16.27–16.35 (d, 1C, -PO-CH2-CH3, 3JC-P = 6.80 Hz), 38.65 (1C, triaz-CH2-carbaz), 58.87–61.27 (d, 1C, -CH-P, 1JC-P = 181.12 Hz), 64.40–64.55 (d, 1C, -PO-CH2-CH3, 2JC-P = 11.3 Hz), 64.49–64.64 (d, 1C, -PO-CH2-CH3, 2JC-P = 11.3 Hz), 108.79, 119.47, 120.41, 123.15, 125.95, 127.63, 128.69, 132.10, 132.70, 140.42 (18C, 6Carom(Ph) + 12Carom(carbaz)), 122.30 (1C, Ct(triaz)), 144.49 (1C,Cq(triaz)), 166.94 (1C, CO). IR (ν(cm−1)): 3050 (N-H), 2850 (Csp3-H), 1630 (C=O), 1250 (P=O), 1050 (P–OEt). Anal. Calcd. for C27H28N5O4P (%): C, 62.66; H, 5.45; N, 13.53; Found (%): C, 62.63; H, 5.47; N, 13.56.
4. Conclusions
In summary, the 1,3-dipolar cycloaddition reaction between azide (1) and the terminal heterocyclic alkyne (2) allows regioselective access to the 1,4-isomer, with excellent yield. The characterization of the structure of the diethyl [(4-{(9H-carbazol-9-yl)methyl}-1H-1,2,3-triazol-1-yl)(benzamido)methyl]phosphonate was performed by different spectroscopic methods and elemental analysis. The heteronuclear 2D NMR allowed us to attribute the structure unequivocally by the observed correlations. This also converges with the data in the literature. The evaluation of the anti-corrosion and biological activities of the synthesized product is under way.
Supplementary Materials
Supplementary Materials of diethyl (azido(benzamido)methyl)phosphonate (1): Figure S1: 1H-NMR spectrum of compound (1), Figure S2: 13C-NMR spectrum of compound (1), Figure S3: Homonuclear 1H-1H spectrum of compound (1), Figure S4: Heteronuclear 1H-13C spectrum of compound (1); Supplementary Materials of 9-(prop-2-yn-1-yl)-9H-carbazole (2): Figure S1: 1H-NMR spectrum of compound (2), Figure S2: 13C-NMR spectrum of compound (2); Supplementary Materials of diethyl [(4-{(9H-carbazol-9-yl)methyl}-1H-1,2,3-triazol-1-yl)(benzamido)methyl]phosphonate (3): Figure S1: Decoupled 31P NMR spectrum of (3), Figure S2: Coupled 31P NMR spectrum of (3), Figure S3: 13C-NMR spectrum of compound (3), Figure S4: 1H-NMR spectrum of compound (3), Figure S5: Homonuclear 1H-1H spectrum of compound (3), Figure S6: Heteronuclear 1H-13C spectrum of compound (3), Figure S7: IR spectrum of compound (3).
Author Contributions
S.A.K.F. performed the experiments; S.A., H.F. and A.A. conceived and designed the experiments; S.A., Y.A., A.N. and A.A. analyzed the data; S.A., Y.A. and A.A. wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
This work was supported by Sidi Mohammed Ben Abdellah University (USMBA) and National Center for Scientific and Technical Research (CNRST).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Elsherbiny, D.A.; Abdelgawad, A.M.; El-Naggar, M.E.; El-Sherbiny, R.A.; El-Rafie, M.H.; El-Sayed, I.E.-T. Synthesis, antimicrobial activity, and sustainable release of novel α-aminophosphonate derivatives loaded Carrageenan Cryogel. Int. J. Biol. Macromol. 2020, 163, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Mucha, A.; Kafarski, P.; Berlicki, Ł. Remarkable potential of the α-aminophosphonate/phosphinate structural motif in Medicinal Chemistry. J. Med. Chem. 2011, 54, 5955–5980. [Google Scholar] [CrossRef] [PubMed]
- Boughaba, S.; Aouf, Z.; Bechiri, O.; Mathe-Allainmat, M.; Lebreton, J.; Aouf, N.-E. H6P2W18O 62·14H2O as an efficient catalyst for the green synthesis of α-aminophosphonates from α-amino acids. Phosphorus Sulfur Silicon Relat. Elem. 2020, 1–8. [Google Scholar] [CrossRef]
- Chiminazzo, A.; Borsato, G.; Favero, A.; Fabbro, C.; McKenna, C.E.; Dalle Carbonare, L.G.; Valenti, M.T.; Fabris, F.; Scarso, A. Diketopyrrolopyrrole bis-phosphonate conjugate: A new fluorescent probe for in vitro bone imaging. Chem. Eur. J. 2019, 25, 3617–3626. [Google Scholar] [CrossRef] [PubMed]
- Rajkoomar, N.; Murugesan, A.; Prabu, S.; Gengan, R.M. Synthesis of methyl piperazinyl-quinolinyl α-aminophosphonates derivatives under microwave irradiation with pd–srtio3 catalyst and their antibacterial and antioxidant activities. Phosphorus Sulfur Silicon Relat. Elem. 2020, 195, 1031–1038. [Google Scholar] [CrossRef]
- Nayab, R.S.; Maddila, S.; Krishna, M.P.; Salam, J.T.; Thaslim, B.S.; Chintha, V.; Wudayagiri, R.; Nagam, V.; Tartte, V.; Chinnam, S. In silico molecular docking and in vitro antioxidant activity studies of novel α-aminophosphonates bearing 6-amino-1,3-dimethyl uracil. J. Recept. Signal Transduct. 2020, 40, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Awad, M.K.; Abdel-Aal, M.F.; Atlam, F.M.; Hekal, H.A. Molecular docking, molecular modeling, vibrational and biological studies of some new heterocyclic α-aminophosphonates. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 206, 78–88. [Google Scholar] [CrossRef]
- Jabli, D.; Dridi, K.; Efrit, M.L. Activité Biologique, réactivité et étude conformationnelle par RMN (1H, 13C, 31P) et DFT des hydrazines phosphonylées vis-à-vis des isothiocyanates. Phosphorus Sulfur Silicon Relat. Elem. 2017, 192, 103–108. [Google Scholar] [CrossRef]
- Chafai, N.; Chafaa, S.; Benbouguerra, K.; Daoud, D.; Hellal, A.; Mehri, M. Synthesis, characterization and the inhibition activity of a new α-aminophosphonic derivative on the corrosion of XC48 carbon steel in 0.5M H2SO4: Experimental and theoretical studies. J. Taiwan Inst. Chem. Eng. 2017, 70, 331–344. [Google Scholar] [CrossRef]
- Benbouguerra, K.; Chafaa, S.; Chafai, N.; Mehri, M.; Moumeni, O.; Hellal, A. Synthesis, spectroscopic characterization and a comparative study of the corrosion inhibitive efficiency of an α-aminophosphonate and schiff base derivatives: Experimental and theoretical investigations. J. Mol. Struct. 2018, 1157, 165–176. [Google Scholar] [CrossRef]
- Kaur, G.; Shamim, M.; Bhardwaj, V.; Gupta, V.K.; Banerjee, B. Mandelic acid catalyzed one-pot three-component synthesis of α-aminonitriles and α-aminophosphonates under solvent-free conditions at room temperature. Synth. Commun. 2020, 50, 1545–1560. [Google Scholar] [CrossRef]
- Maestro, A.; Marigorta, E.M.; Palacios, F.; Vicario, J. α-Iminophosphonates: Useful intermediates for enantioselective synthesis of α-aminophosphonates. Asian J. Org. Chem. 2020, 9, 538–548. [Google Scholar] [CrossRef]
- Tripolszky, A.; Tóth, E.; Szabó, P.T.; Hackler, L.; Kari, B.; Puskás, L.G.; Bálint, E. Synthesis and in vitro cytotoxicity and antibacterial activity of novel 1,2,3-triazol-5-yl-phosphonates. Molecules 2020, 25, 2643. [Google Scholar] [CrossRef] [PubMed]
- Elachqar, A.; El Hallaoui, A.; Roumestant, M.L.; Viallefont, P. Synthesis of heterocyclic α-aminophosphonic acids. Synth. Commun. 1994, 24, 1279–1286. [Google Scholar] [CrossRef]
- Aouine, Y.; Faraj, H.; Alami, A.; El Hallaoui, A.; Elachqar, A.; El Hajji, S.; Kerbal, A.; Labriti, B.; Martinez, J.; Rolland, V. Synthesis of new triheterocyclic compounds, precursors of biheterocyclic amino acids. J. Mar. Chim. Heterocycl. 2008, 7, 44–49. [Google Scholar]
- Tripolszky, A.; Németh, K.; Szabó, P.T.; Bálint, E. Synthesis of (1,2,3-triazol-4-yl)methyl phosphinates and (1,2,3-triazol-4-yl)methyl phosphates by copper-catalyzed azide-alkyne cycloaddition. Molecules 2019, 24, 2085. [Google Scholar] [CrossRef]
- Song, W.; Zheng, N.; Li, M.; Ullah, K.; Zheng, Y. Rhodium(I)-catalyzed azide-alkyne cycloaddition (RhAAC) of internal alkynylphosphonates with high regioselectivities under mild conditions. Adv. Synth. Catal. 2018, 360, 2429–2434. [Google Scholar] [CrossRef]
- Huisgen, R. Chimie de la Cycloaddition 1,3-Dipolaire; Wiley: New York, NY, USA, 1984; p. 1. [Google Scholar]
- Bentama, A.; El Hadrami, E.M.; El Hallaoui, A.; Elachqar, A.; Lavergne, J.-P.; Roumestant, M.-L.; Viallefont, P.H. Synthesis of new α-heterocyclic α-amino esters. Amino Acids 2003, 24, 423–426. [Google Scholar] [CrossRef]
- Achamlale, S.; Elachqar, A.; El Hallaoui, A.; El Hajji, S.; Alami, A.; Roumestant, M.L.; Viallefont, Ph. Synthesis of biheterocyclic α-aminophosphonic acid derivatives. Phosphorus Sulfur Silicon 1998, 140, 103–111. [Google Scholar] [CrossRef]
- Boukallaba, K.; Elachqar, A.; El Hallaoui, A.; Alami, A.; El Hajji, S.; Labriti, B.; Rolland, V. Synthesis of new α-heterocyclic α-aminophosphonates. Phosphorus Sulfur Silicon Relat. Elem. 2006, 181, 819–823. [Google Scholar] [CrossRef]
- Boukallaba, K.; Elachqar, A.; El Hallaoui, A.; Alami, A.; El Hajji, S.; Labriti, B.; Rolland, V. Synthesis of α-heterocyclic α-aminophosphonates, part II: Morpholine, piperidine, pyrrolidine, tetrahydrofurylmethylamine, N-benzyl-N-methylamine, and aniline derivatives. Phosphorus Sulfur Silicon Relat. Elem. 2007, 182, 1045–1052. [Google Scholar] [CrossRef]
- Achamlale, S.; Alami, A.; Aouine, Y. Structure assignment of N-protected 2-(1H-1,2,3-triazol-1-yl)-glycine derivatives by chemical and spectroscopic methods. J. Mar. Chim. Heterocycl. 2019, 18, 61–69. [Google Scholar]
- Vorobyeva, D.V.; Karimova, N.M.; Vasilyeva, T.P.; Osipov, S.N.; Shchetnikov, G.T.; Odinets, I.L.; Röschenthaler, G.-V. Synthesis of functionalized α-CF3-α-aminophosphonates via Cu(I)-catalyzed 1,3-dipolar cycloaddition. J. Fluor. Chem. 2010, 131, 378–383. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).