N-{2-[(3-Oxo-1,3-dihydro-2-benzofuran-1-yl)acetyl]phenyl}acetamide
Abstract
1. Introduction
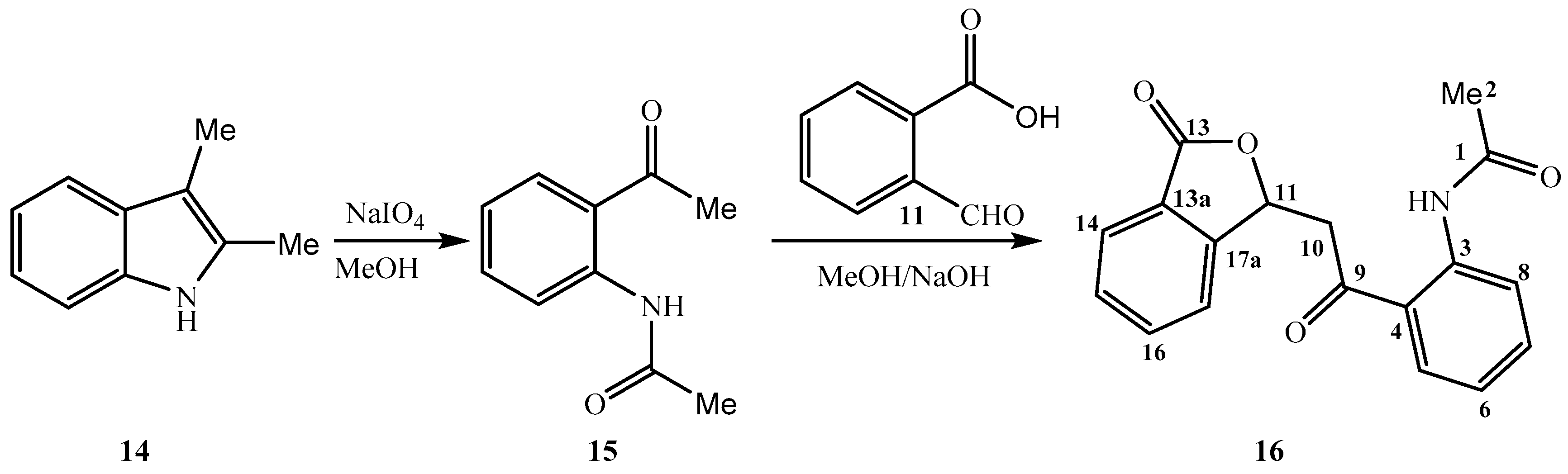
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. Synthesis of N-{2-[(3-Oxo-1,3-dihydro-2-benzofuran-1-yl)acetyl]phenyl}acetamide 16
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zou, S.; Wang, Z.; Wang, J.; Wei, G.; Wang, W.; Zang, Y.; Zeng, F.; Chen, K.; Liu, J.; Wang, J.; et al. Five new aza-epicoccone derivatives from Aspergillus flavipes. Fitoterapia 2018, 124, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.-D.; Dong, Z.-J.; Liu, J.-K.; Yang, L.-M.; Wang, R.-R.; Zheng, Y.-T.; Lu, Y.; Wu, Y.-S.; Zheng, Q.-T. Concentricolide, an anti-HIV agent from the ascomycete Daldinia concentrica. Helv. Chim. Acta 2006, 89, 127–133. [Google Scholar] [CrossRef]
- Rodrigues, M.P.; Tomaz, D.C.; de Souza, L.Â.; Onofre, T.S.; de Menezes, W.A.; Almeida, J.; Suarez, A.M.; de Almeida, M.R.; da Silva, A.M.; Costa, G.; et al. Synthesis of cinnamic acid derivatives and leishmanicidal activity against Leishmania braziliensis. Eur. J. Med. Chem. 2019, 183, 111688. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Luo, B.; Luo, Z.; Zhang, L.; Fan, J.W.; Tang, L.; Li, Y. Synthesis and antifungal activities of 3-substituted phthalide derivatives. Z. Naturforsch. B 2019, 74, 811–818. [Google Scholar] [CrossRef]
- Teixeira, R.R.; Pereira, W.L.; Tomaz, D.C.; de Oliveira, F.M.; Giberti, S.; Forlani, G. Synthetic analogues of the natural com-pound cryphonectric acid interfere with photosynthetic machinery through two different mechanisms. J. Agric. Food Chem. 2013, 61, 5540–5549. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Shi, X.; Li, J.; Hou, Z.; Song, Z.; Su, X.; Peng, D.; Wang, F.; Yu, Y.; Zhao, G. Nickel-catalized amidoalkylation reaction of γ-hidroxy lactams: An access to 3-substituted isoindolinones. ACS Omega 2019, 4, 19420–19436. [Google Scholar] [CrossRef] [PubMed]
- Sashidhara, K.V.; Singh, L.R.; Palnati, G.R.; Avula, S.R.; Kant, R. A catalyst-free one pot protocol for the construction of sub-stituted isoindolinones under sustainable conditions. Synlett 2016, 27, 2384–2390. [Google Scholar] [CrossRef][Green Version]
- Lübbers, T.; Angehrn, P.; Gmünder, H.; Herzig, S. Design, synthesis, and structure-activity relationship studies of new phenolic DNA gyrase inhibitors. Bioorg. Med. Chem. Lett. 2007, 17, 4708–4714. [Google Scholar] [CrossRef] [PubMed]
- Bassin, J.P.; Anagani, B.; Benham, C.; Goyal, M.; Hashemian, M.; Gerhard, U. Synthesis and spectral characterization of ben-zo-[6,7][1,5]diazocino[2,1-a]isoindol-12-(14H)-one derivatives. Molecules 2016, 21, 967. [Google Scholar] [CrossRef] [PubMed]
- Abonia, R.; Cuervo, P.; Insuasty, B.; Quiroga, J.; Nogueras, M.; Cobo, J.; Meier, H.; Lotero, E. An Amberlyst-15® Mediated Synthesis of New Functionalized Dioxoloquinolinone Derivatives. TOOCJ 2008, 2, 26–34. [Google Scholar] [CrossRef]
- Lee, J.; Jung, H. An Efficient Synthesis of 2,3-Dihydro-2-phenyl-4-quinolones from 2’-Aminoacetophenones. J. Korean Chem. Soc. 2007, 51, 106–110. [Google Scholar] [CrossRef]
- Yaeghoobi, M.; Frimayanti, N.; Chee, C.F.; Ikram, K.K.; Najjar, B.O.; Zain, S.M.; Abdullah, Z.; Wahab, H.; Rahman, N.A. QSAR, in silico docking and in vitro evaluation of chalcone derivatives as potential inhibitors for H1N1 virus neuraminidase. Med. Chem. Res. 2016, 25, 2133–2142. [Google Scholar] [CrossRef]
- Pan, G.F.; Su, L.; Zhang, Y.L.; Guo, S.H.; Wang, Y.Q. Organocatalytic one-pot asymmetric synthesis of 2-aryl-2,3-dihydro-4-quinolones. RSC Adv. 2016, 6, 25375–25378. [Google Scholar] [CrossRef]
- Ullah, A.; Ansari, F.L.; ul-Haq, I.; Nazir, S.; Mirza, B. Combinatorial synthesis, lead identification, and antitumor study of a chalcone-based positional-scanning library. Chem. Biodivers. 2007, 4, 203–214. [Google Scholar] [CrossRef]

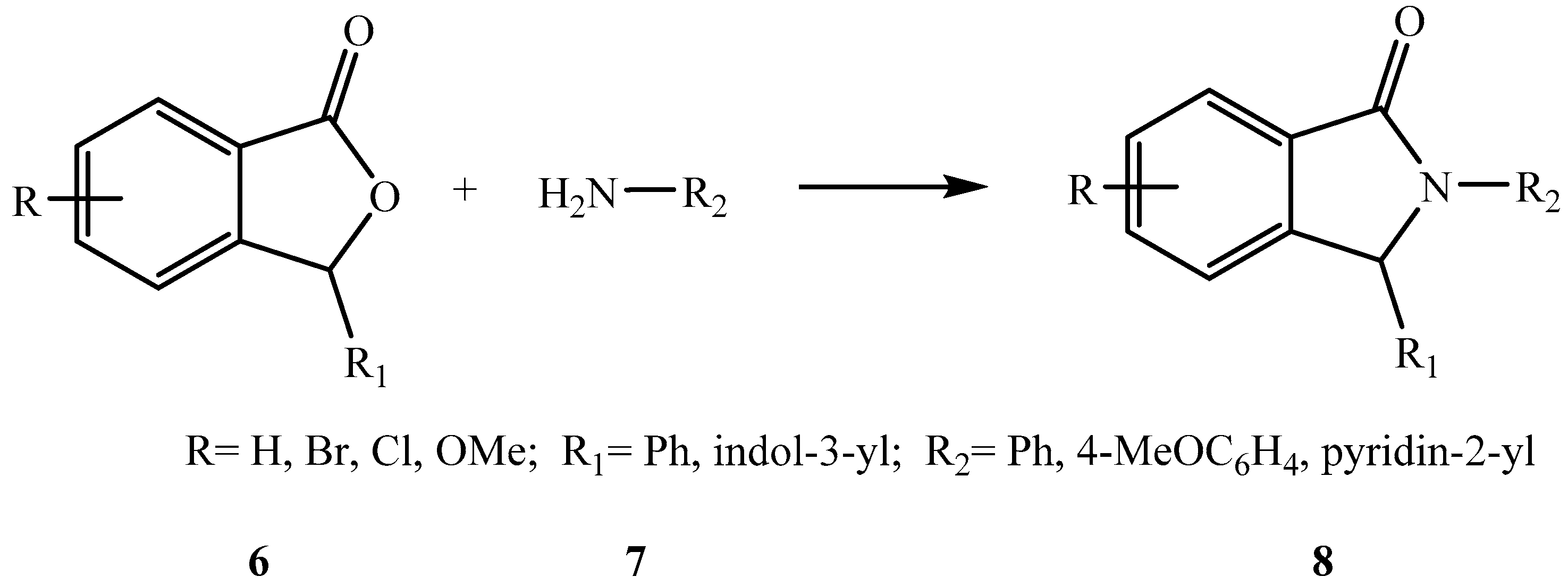


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez, R.; León, O.; Quiroga, F.; Cifuentes, J. N-{2-[(3-Oxo-1,3-dihydro-2-benzofuran-1-yl)acetyl]phenyl}acetamide. Molbank 2021, 2021, M1244. https://doi.org/10.3390/M1244
Rodríguez R, León O, Quiroga F, Cifuentes J. N-{2-[(3-Oxo-1,3-dihydro-2-benzofuran-1-yl)acetyl]phenyl}acetamide. Molbank. 2021; 2021(3):M1244. https://doi.org/10.3390/M1244
Chicago/Turabian StyleRodríguez, Ricaurte, Omar León, Felipe Quiroga, and Jonnathan Cifuentes. 2021. "N-{2-[(3-Oxo-1,3-dihydro-2-benzofuran-1-yl)acetyl]phenyl}acetamide" Molbank 2021, no. 3: M1244. https://doi.org/10.3390/M1244
APA StyleRodríguez, R., León, O., Quiroga, F., & Cifuentes, J. (2021). N-{2-[(3-Oxo-1,3-dihydro-2-benzofuran-1-yl)acetyl]phenyl}acetamide. Molbank, 2021(3), M1244. https://doi.org/10.3390/M1244






