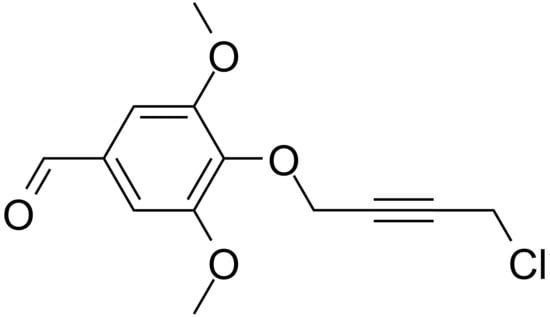
1-(4-Formyl-2,6-dimethoxyphenoxy)-4-chlorobut-2-yne
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Materials
Funding
Data Availability Statement
Conflicts of Interest
References
- Chai, Q.-Y.; Yang, Z.; Lin, H.-W.; Han, B.-N. Alkynyl-Containing Peptides of Marine Origin: A Review. Mar. Drugs 2016, 14, 216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, F.H.; Putoczki, T.L.; Stylli, S.S.; Luwor, R.B. Ponatinib: A novel multi-tyrosine kinase inhibitor against human malignancies. OncoTargets Ther. 2019, 12, 635–645. [Google Scholar] [CrossRef] [Green Version]
- Li, K.; Wong, N.K.; Strauss, M.J.; Evans, A.M.; Matsumoto, M.; Dichtel, W.R.; Adronov, A. Postsynthetic Modification of a Covalent Organic Framework Achieved via Strain-Promoted Cycloaddition. J. Am. Chem. Soc. 2021, 143, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Schrettl, S.; Contal, E.; Hoheisel, T.N.; Fritzsche, M.; Balog, S.; Szilluweit, R.; Frauenrath, H. Facile synthesis of oligoyne amphiphiles and their rotaxanes. Chem. Sci. 2015, 6, 564–574. [Google Scholar] [CrossRef] [Green Version]
- Alabugin, I.V.; Gonzalez-Rodriguez, E.; Kawade, R.K.; Stepanov, A.A.; Vasilevsky, S.F. Alkyne as Synthetic Equivalents of Ketones and Aldehydes: A Hidden Entry into Carbonyl Chemistry. Molecules 2019, 24, 1036. [Google Scholar] [CrossRef] [Green Version]
- Matsuda, I.; Ogiso, A.; Sato, S.; Izumi, Y. An Efficient Silylformylation of Alkynes Catalyzed by Rh4(CO)12. J. Am. Chem. Soc. 1989, 111, 2332–2333. [Google Scholar] [CrossRef]
- Lei, Y.-R.; Liang, J.-Y.; Wang, Y.-J.; Chen, Z. Preparation of vincinal hetero 1,2-dihalo-olefins by using aqueous hydrohalic acid. Tetrahedron Lett. 2021, 70, 152968. [Google Scholar] [CrossRef]
- Zhao, M.; Liu, J.-L.; Liu, H.-F.; Chen, J.; Zhou, L. Construction of Bisbenzopyrone via N-Heterocyclic Carbene Catalyzed Intramolecular Hydroacylation-Stetter Reaction Cascade. Org. Lett. 2018, 20, 2676–2679. [Google Scholar] [CrossRef]
- Pribut, N.; Veale, C.G.; Basson, A.E.; van Otterlo, W.A.L.; Pelly, S.C. Application of the Huisgen cycloaddition and ‘click’ reaction toward various 1,2,3-triazoles as HIV non-nucleoside reverse transcriptase inhibitors. Bioorg. Med. Chem. Lett. 2016, 26, 3700–3704. [Google Scholar] [CrossRef]
- Ma, J.; Ding, S. Transition Metal-Catalyzed Cycloaddition of Azides with Internal Alkynes. Asian J. Org. Chem. 2020, 9, 1872–1888. [Google Scholar] [CrossRef]
- Lutz, J.F. 1,3-Dipolar Cycloadditions of Azides and Alkynes: A Universal Ligation Tool in Polymer and Materials Science. Angew. Chem. Int. Ed. 2007, 46, 1018–1025. [Google Scholar] [CrossRef] [PubMed]
- Fantoni, N.Z.; El-Sagheer, A.H.; Brown, T. A Hitchhiker’s Guide to Click-Chemistry with Nucleic Acids. Chem. Rev. 2021, 121, 7122–7154. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.S.; Jourdain, I.; Knorr, M.; Boullanger, S.; Brieger, L.; Strohmann, C. Heterodinuclear Diphosphane-Bridged Iron-Platinum Diyne Complexes as Metalloligands for the Assembly of Polymetallic Systems (Fe, Pt, Co). J. Clust. Sci. 2019, 30, 1211–1225. [Google Scholar] [CrossRef]
- Jourdain, I.; Knorr, M.; Strohmann, C.; Unkelbach, C.; Rojo, S.; Gomez-Iglesias, P.; Villafane, F. Reactivity of Silyl-Substituted Iron−Platinum Hydride Complexes toward Unsaturated Molecules: 4. Insertion of Fluorinated Aromatic Alkynes into the Platinum−Hydride Bond. Synthesis and Reactivity of Heterobimetallic Dimetallacylopentenone, Dimetallacyclobutene, μ-Vinylidene, and μ2-σ-Alkenyl Complexes. Organometallics 2013, 32, 5343–5359. [Google Scholar]
- Wong, Y.-S.; Ng, M.; Yeung, M.C.-L.; Yam, V.W.-W. Platinum(II)-Based Host-Guest Coordination-Driven Supramolecular Co-Assembly Assisted by Pt•••Pt and π−π Stacking Interactions: A Dual-Selective Luminescence Sensor for Cations and Anions. J. Am. Chem. Soc. 2021, 143, 973–982. [Google Scholar] [CrossRef] [PubMed]
- Pinto, A.; Spigolon, G.; Gavara, R.; Zonta, C.; Licini, G.; Rodriguez, L. Tripodal gold(I) polypyridyl complexes and their Cu+ and Zn2+ heterometallic derivatives. Effects on luminescence. Dalton Trans. 2020, 49, 14613–14625. [Google Scholar] [CrossRef]
- Ibrahim, M.N.M.; Sriprasanthi, R.B.; Shamsudeen, S.; Adam, F.; Bhawani, S.A. A Concise Review of the Natural Existence, Synthesis, Properties, and Applications of Syringaldehyde. BioResources 2012, 7, 4377–4399. [Google Scholar]
- Hillard, J.; Reddy, K.V.; Majumbar, K.C.; Thyagarajan, B.S. A New Synthesis of 2,3-Disubstituted Indoles. J. Heterocycl. Chem. 1974, 11, 369–375. [Google Scholar] [CrossRef]
- Arnone, A.; Cardillo, R.; Nasini, G.; Vajna de Pava, O. Two Cinnamic Allenic Ethers from the Fungus Clitocybe Eucalyptorum. Phytochemistry 1993, 32, 1279–1281. [Google Scholar] [CrossRef]
- Canceill, J.; Collet, A.; Gottarelli, G.; Palmieri, P. Synthesis and Exciton Optical Activity of D3-Cryptophanes. J. Am. Chem. Soc. 1987, 109, 6454–6464. [Google Scholar] [CrossRef]
- Padial, N.M.; Hernandez-Cervantes, C.; Munoz-Bascon, J.; Roldan-Molina, E.; Garcia-Martinez, M.; Ruiz-Muelle, A.B.; Rosales, A.; Alvarez-Corral, M.; Munoz-Dorado, M.; Rodriguez-Garcia, I.; et al. Ti-Catalyzed Synthesis of Exocyclic Allenes on Oxygen Heterocycles. Eur. J. Org. Chem. 2017, 7, 639–645. [Google Scholar] [CrossRef]
- Michaux, J.; Poirot, R.; Einhorn, J.; Bessières, B. Co(I) catalyzed yne-ene-yne [2+2+2] cycloaddition: Synthesis of highly strained pentacyclic bis-lactones. A new access to tetraaryl N-hydroxyphtalimides. Tetrahedron Lett. 2014, 55, 2849–2852. [Google Scholar] [CrossRef]
- Yusuf, M.; Samdhian, V.; Jain, P. Synthesis, Characterization, and Antimicrobial Studies of New Rigid Linker-Based Bispyrazolines. J. Heterocycl. Chem. 2015, 52, 260–266. [Google Scholar] [CrossRef]
- Yusuf, M.; Manpreet, K.; Jain, P. Synthesis and Antimicrobial Evaluations of 1,3,4-Thiadiazoline-based Bisheterocyclics. J. Heterocycl. Chem. 2015, 52, 692–700. [Google Scholar] [CrossRef]
- Kiehlmann, E.; Li, E.P.-M.; Millar, J.G. The Claisen rearrangement of 1,4-bis(m-methoxyphenoxy-)2-butyne. Can. J. Chem. 1986, 64, 1989–1997. [Google Scholar] [CrossRef]
- Toda, F.; Nakagawa, M. Cyclic Acetylenes. V. Syntheses and Properties of Strained Cyclic Ethers of o,o’-Dihydroxydiphenyldiacetylene. Bull. Chem. Soc. Jpn. 1961, 34, 862–874. [Google Scholar] [CrossRef]
- Yusuf, M.; Solanki, I.; Jain, P. Synthesis and antimicrobial studies of new N,N′-[5,5′-{2,2′-(bis-alkoxy)bis(2,1-phenylene)]bis(4-acetyl-4,5-dihydro-1,3,4-thiadiazole-5,2-diyl)]diacetamide. J. Chem. Sci. 2012, 124, 703–715. [Google Scholar] [CrossRef] [Green Version]
- Yusuf, M.; Saloni, T. Multicomponent synthesis of new bis(pyranopyrazoles) and their antimicrobial-antioxidant evaluations. J. Serb. Chem. Soc. 2019, 84, 119–128. [Google Scholar] [CrossRef] [Green Version]
- Yusuf, M.; Saloni, T. Bis(4,5-dihydropyrazole) Derivatives: Synthesis, Characterization and Antimicrobial-Antioxidant Evaluations. Asian J. Chem. 2018, 30, 2097–2102. [Google Scholar] [CrossRef]
- Naidu, B.N.; Wang, T.; Peese, K.; Yin, Z.; Zhang, Z.; Kadow, J.F.; Patel, M. Pyrazolopyrimidine Macrocycles as Inhibitors of Human Immunodeficiency Virus Replication. U.S. Patent WO2015126743, 20 August 2015. [Google Scholar]
- Behm, R.; Gloeckner, C.; Grayson, M.A.; Gross, M.L.; Gokel, G.W. Pyxophanes: Selective gas phase ion complexation by 1,6,13,18-tetraoxa[6.6]paracyclophane-3,15-diyne. Chem. Commun. 2000, 23, 2377–2378. [Google Scholar] [CrossRef]
- Eid, C.N.; Knobler, C.B.; Gronbeck, D.A.; Cram, D.J. Binding Properties of Two New Hemicarcerands Whose Hemicarceplexes Undergo Chemical Reactions without Guest Release. J. Am. Chem. Soc. 1994, 116, 8506–8515. [Google Scholar] [CrossRef]
- Lustenberger, P.; Martinborough, E.; Mordasini Denti, T.; Diederich, F. Geometrical optimization of 1,1′-binaphtalene receptors for enantioselective molecular recognition of excitatory amino acid derivatives. J. Chem. Soc. Perkin Trans. 1998, 4, 747–761. [Google Scholar] [CrossRef]
- Canceill, J.; Collet, A. Two-step Synthesis of D3 and C3h Cryptophanes. J. Chem. Soc. Chem. Commun. 1988, 9, 582–584. [Google Scholar] [CrossRef]
- Thyagarajan, B.S.; Balasubramanian, K.K.; Bhima Rao, R. Studies in Claisen Rearrangement-II 2-Butyn-1,4-diyl Bis-(aryl ethers). Tetrahedron 1965, 21, 2289–2295. [Google Scholar] [CrossRef]
- Davis, A.C.; Hunter, R.F. Polymerisation of Acetylene Derivatives. III. Polymerization of a Dimer of 4-Penten-2-yn-1-ol and Attempted Polymerisation and Copolymerisation with Styrene of 1:1:3:3:6-Pentamethyl-4-isopropenylisocoumaran, 1:4-Di-p-chlorophenoxy-2-butyne, and 2-Methyl-2-isopropenyl-4-methyloldioxolan. J. Appl. Chem. 1959, 9, 660–664. [Google Scholar]
- Rudorf, W.-D.; Janietz, D. Intramolekulare 1,3-dipolare Cycloadditionen von Arylaziden mit Alkinylsubstituenten. J. Prakt. Chem. 1987, 1, 55–61. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Husson, J. 1-(4-Formyl-2,6-dimethoxyphenoxy)-4-chlorobut-2-yne. Molbank 2021, 2021, M1252. https://doi.org/10.3390/M1252
Husson J. 1-(4-Formyl-2,6-dimethoxyphenoxy)-4-chlorobut-2-yne. Molbank. 2021; 2021(3):M1252. https://doi.org/10.3390/M1252
Chicago/Turabian StyleHusson, Jérôme. 2021. "1-(4-Formyl-2,6-dimethoxyphenoxy)-4-chlorobut-2-yne" Molbank 2021, no. 3: M1252. https://doi.org/10.3390/M1252
APA StyleHusson, J. (2021). 1-(4-Formyl-2,6-dimethoxyphenoxy)-4-chlorobut-2-yne. Molbank, 2021(3), M1252. https://doi.org/10.3390/M1252